By YAN Fusheng (Staff Reporter)
Why different species have different numbers of chromosomes? Let's take a peek into the cute little mouse below whose two chromosomes are fused artificially in the lab, and this may give you something to ponder.

An artwork illustrates the chromosome fusion in mice. (Credit: GU Deng-er)
Reported online in Cell Research on September 21, 2022, a team led by CAS Member Prof. LI Jinsong from the Center for Excellence in Molecular and Cell Science (Shanghai Institute of Biochemistry and Cell Biology), Chinese Academy of Sciences, has been successful in fusing two chromosomes by head-to-head in mice.
These modified mice carry 19 pairs of chromosomes, instead of 20 pairs as in normal mice, and can produce viable offspring that carry a fused chromosome. Moreover, LI and his team also reveal a cell’s robustness in coping with chromosomal rearrangements, which could explain why this planet is teeming with millions of species.
Questions to Be Answered
Species can be defined by the size, shape, and number of their chromosomes, or as termed as the ‘karyotype’. Its stability usually dominates one individual’s survival because variations in the number and structure of chromosomes are usually accompanied by adverse effects. However, the karyotype change also fuels species evolution, i.e., the creation of new species.
Based on systems biology research, about 3 to 4 million years ago, two ancestral acrocentric chromosomes corresponding to chromosomes 2a and 2b in chimpanzees (2n =48, where n is a single set of chromosomes) fused by head-to-tail into human chromosome 2 (2n=46). This change may have directly caused reproductive isolation between the ancestors of humans and the ancestors of chimpanzees, heralding the rise of the planet of humans. However, the exact mechanism by which this event occurred remains elusive.
The lab mouse (Mus musculus) has maintained a standard 40-chromosome karyotype after more than 100 years of artificial breeding. Intriguingly, the telocentric chromosomes, which are known to be relatively unstable, prevail in mice.
However, wild mice have a wide range of subspecies with different chromosomal Robertsonian (Rob) fusions (sometimes referred to as Robertsonian translocations, or centric fusions), a type of head-to-head fusion occurs between two telocentric chromosomes, which is one of the most frequent events in mammalian karyotype evolution. Interestingly, the distribution of Rob fusion types in nature is very uneven. Rob (2, 4) (fusion of chromosomes 2 and 4) and Rob (5, 15) fusion types prevail in Asia, Europe and America. In contrast, fusion types such as Rob (1, 13) and Rob (2, 9) have not been reported.
These phenomena lead to a series of questions. How does chromosome fusion happen? Why does mouse evolution have a preference to telocentric chromosomes that are relatively unstable? Do different types of chromosome fusions affect the life activities of cells and individuals? How can a mouse’s one-armed chromosomes be remodeled into two-armed chromosomes that are more like human chromosomes?
Forerunners
In 2018, a team of Chinese scientists genetically ‘stitched’ yeast’s 16 chromosomes into one single giant chromosome using head-to-tail fusion. Surprisingly, the life of modified yeasts is almost all going well. This feat opened a new window for studying chromosome rearrangement or tweaking an organism’s karyotype.
However, it was a daunting task to repeat this feat in mammals. Luckily, a technology termed “sperm-like stem cell”, established independently by LI’s team and another team led by CAS member ZHOU Qi from the Institute of Zoology in 2012, illuminated the possibility of individually remodeling a mammal’s chromosomes.
On August 26, 2022, a team led by ZHOU and Prof. LI Wei from the CAS Institute of Zoology reported in Science their success in obtaining three mice carrying 19 pairs of chromosomes through using a similar head-to-tail fusion method in sperm-like stem cells.
One Step Further
Using a new scheme of genetic tweak targeting the mouse centromeres, Prof. LI Jinsong and his team transfected the centromere-targeted cleavage system into mouse sperm-like stem cells and achieved Robertsonian fusions by head-to-head in the lab. This study successfully recaptures the chromosomal rearrangement events that occurred in nature.
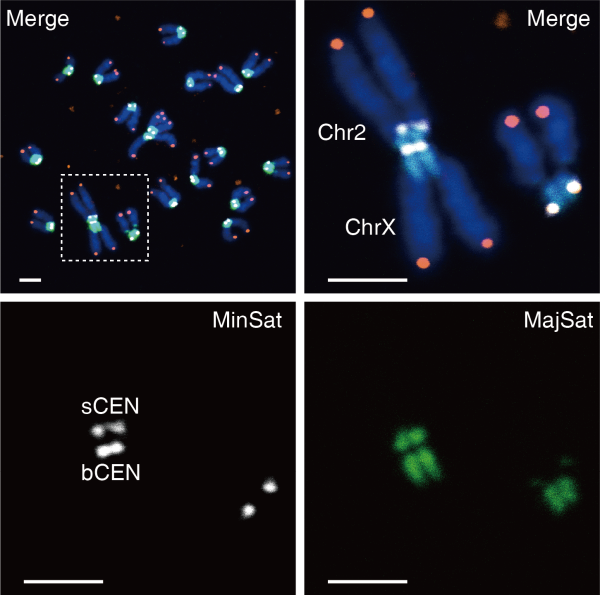
Mouse chromosome 2 (Chr2) in a sperm-like stem cell fused with chromosome X (ChrX) head-to-head into a double-armed chromosome. The red dots are telomeres. The white parts are centromeres, and the green is a pericentromeric region. (Image by LI’s team)
From 1,128 monoclonal cell lines transfected with centromeric cleavage components, the researchers established ten haploid cell lines with stable 19 chromosomes, 9 of which maintained genomic ploidy balance.
Chromosome centromeres break and fuse to form a new centromere. The researchers found that all double-armed chromosomes formed after Rob fusion with mouse chromosome 2 (Chr2) have two independent centromeres. Further analysis shows that mouse Chr2 has two terminal centromeres, unlike other chromosomes. Two centromeres at one chromosome is a bad omen for achieving a stable chromosomal separation during mitosis.
The good news is one of them is inactive. This finding implies another story about a special centromere formation event in the mouse Chr2 chromosome, which would begin with “A long time ago, there was a mouse….”
Intriguingly, the research also found that the mating of mice with 19 pairs of chromosomes and that with 20 pairs can produce fertile offspring.
“This shows that simple chromosomal rearrangement by fusing two chromosomes does not suffice for reproductive isolation. It seems the establishment of reproductive isolation is a cumulative process,” explained Prof. LI Jinsong, the corresponding author of this study.
Chromosome Robustness
LI and his team then looked into what effect chromosome fusion have on the cell’s activities.
The fusion brings two chromosomes in proximity. Though researchers picked up an increased interplay between two involved chromosomes, chromosome fusion has a minimal effect on the cell’s whole-level gene expression profile, indicating a certain degree of homeostasis or robustness in the cell’s genome organization.
Sperm-like stem cells can replace sperm to ‘fertilize’ oocytes. After injecting chromosome fusion-engineered sperm-like stem cells into eggs, researchers can create healthy ‘semi-cloned’ mice in the lab. They successfully established four independent homozygous chromosome fusion mouse strains and used these mice to mate and breed offspring that inherit the changed karyotypes.
Next, the researchers created sperm-like stem cells with multiple fused chromosomes. They observed an enhanced perturbation in the cell’s gene expression profile as the number of fusion events increased. Using this scheme, they can quickly generate semi-cloned mice models with multiple fusions in lab.
The homozygous male with 19 pairs of chromosomes and its cubs (upper). Semi-cloned female pups (in black) carry three double-armed chromosomes (bottom) with their foster mother.
A New Era
This study suggested that chromosome fusion caused by centromere breakage fuels chromosomal evolution, and the robustness of eukaryotic genome rearrangement is an essential keystone for this course.
It also provides a feasible technical route for the modification of chromosome structure in mammals and the creation of new animal karyotype subspecies.
Based on this technique platform, LI and his team could simulate human genetic diseases caused by abnormal Robertsonian fusions occurred between the human chromosomes 13/14/15/21, human chromosomal inversion diseases, and other chromosomal abnormalities such as human trisomy (Down syndrome, Kerr syndrome, Edward syndrome, etc.).
“Sperm-like stem cell-mediated semi-cloning technology, as a powerful genetic carrier for connecting cells and individuals, will have great vitality in the future with the support of synthetic biology and systems biology,” says Prof. LI. “Perhaps one day in the future, looking back in the history of science, we can define 2022 as the first year of genome remodeling at the chromosome scale in mammals.”
References
Yang, H., Shi, L., Wang, B. A., et al. (2012). Generation of genetically modified mice by oocyte injection of androgenetic haploid embryonic stem cells. Cell, 149(3), 605-617. doi:10.1016/j.cell.2012.04.002
Li, W., Shuai, L., Wan, H., et al. (2012). Androgenetic haploid embryonic stem cells produce live transgenic mice. Nature, 490(7420), 407-411. doi:10.1038/nature11435
Shao, Y., Lu, N., Wu, Z., et al. (2018). Creating a functional single-chromosome yeast. Nature, 560(7718), 331-335. doi:10.1038/s41586-018-0382-x
Wang, L. B., Li, Z. K., Wang, L. Y., et al. (2022). A sustainable mouse karyotype created by programmed chromosome fusion. Science, 377(6609), 967-975. doi:10.1126/science.abm1964
Zhang, X. M., Yan, M., Yang, Z., et al. (2022). Creation of artificial karyotypes in mice reveals robustness of genome organization. Cell Research. doi:10.1038/s41422-022-00722-x

