By SONG Jianlan (Staff Reporter)
In our conversation with Prof. CHEN Lingling on the “dark matter” in the genome, namely the non-coding RNAs, we got to know two classes of long non-coding RNAs (lncRNAs) in close association with Prader-Willi syndrome (PWS). Each with a snoRNA cap on its either end, they fall in the sno-lncRNA family, a group of lncRNAs identified by CHEN’s group in their earlier research. Here we are meeting another member of the family, SLERT, a rescuer who liberates molecules of RNA polymerase I (Pol I) from a suffocating siege in the nucleus.
Releasing the latter to produce ribosomal RNAs (rRNAs) is required for the very beginning of the assembly line of protein synthesis. Of note, dysregulated rRNA production is associated with uncontrolled cell proliferation, including human cancers.

A schematic illustration of SLERT, the knight who raises the siege of RNA polymerase I and activates ribosomal RNA (rRNA) production to ensure the assembly of protein synthesis machineries. (Image by CHEN LL)
Since the Human Genome Program, more and more evidence demonstrates that non-coding RNAs, formerly deemed as “junk sequences” in the genome, pervasively mediate very important regulative processes in our cells, without coding any functional peptides or proteins. More recent studies in this field further unveiled their crucial roles in some regulative pathways pertinent to human diseases, including cancers. A newly identified long non-coding RNA with hairpin-like snoRNA tails, the SLERT, was lately brought to light by CAS scientists as another example.
In their recent work published in Cell in May 2017, a team led by Prof. CHEN Lingling at the Shanghai Institute of Biochemistry and Cell Biology (SIBCB), CAS reported a sno-lncRNA able to enhance the transcription of precursors of ribosomal RNAs (pre-rRNAs), and regulate the differentiated expression of rRNAs in the nucleolus, a sub-structure in the nucleus. Their work revealed in detail how this sno-lncRNA named “Sno-LncRNA Enhances Ribosomal RNA Transcription” (SLERT) regulates the transcription of pre-rRNAs, and meanwhile unveiled an interesting episode in nucleolus.
Why is the regulation of transcription of pre-rRNAs so important as to impact the occurrence of cancers? An answer might be, this process heads a long assembly line for protein synthesis in eukaryotic cells: Every error occurring at this stage could escalate into serious consequences in downstream assembly of protein synthesis machineries and thus defects of protein synthesis in physiological processes.
Tricky Pre-rRNA Transcription
Transcribed from rDNAs by RNA polymerase I (Pol I), pre-rRNAs undergo some modification and processing to mature into rRNAs, and further form ribosomes in the cytoplasm by binding to various proteins. The main role of ribosomes is to recognize the right amino acid for every genetic code transcribed to the messenger RNA and load them onto the protein skeleton, securing appropriate synthesis of proteins. As the precondition of this process, the transcription of pre-rRNAs constitutes the very beginning of protein production, therefore any disorder in its regulation will have an impact on all downstream stages of the whole protein assembly line.
That is why dysregulated transcription of pre-rRNAs could lead to a serious aftermath: insufficient transcription impedes the formation of ribosomes and hence downstream proteins, leading to anemia of bone-marrow failure; and over transcription, on the contrary, results in external cell proliferation and causes cancers.
In their experiments, CHEN’s team observed that SLERT, a human-specific sno-lncRNA, plays its role via regulating a ring-shape structure formed by DDX21 proteins in the nucleolus that normally act to repress Pol I transcription in human cells.
The Nucleolus
The nucleolus is exactly where the pre-rRNAs are synthesized: rDNAs are transcribed here by Pol I to produce pre-rRNAs. This nuclear structure plays a key role in coordinating the biogenesis of ribosome, and the level of ribosome production is mainly determined by the activity of Pol I.
Interestingly, SLERT, the leading star of this episode, is generated from TBRG4, a gene located outside the nucleoli; only via the aid from its snoRNA-ends can it transfer to the nucleoli to carry out its function.
Noted that SLERT mainly accumulates at the nucleolus, the researchers wondered if it promotes pre-rRNA transcription. To test this speculation, the researchers designed a rigorous scheme of experiments to verify the exact roles of SLERT and dissect the rRNA production on transcription/processing level. To exclude the influence from proteins expressed by TBRG4, the parental gene of SLERT, they accurately knocked out corresponding fragments of SLERT from the transcribed genome using CRISPR/CAS9 system. As expected, without changing the level of TBRG4 protein expression, they observed remarkable reduction in nascent pre-rRNA production, suggesting decreased level of pre-rRNA transcription in SLERT knockout cells, compared with wild type cells.
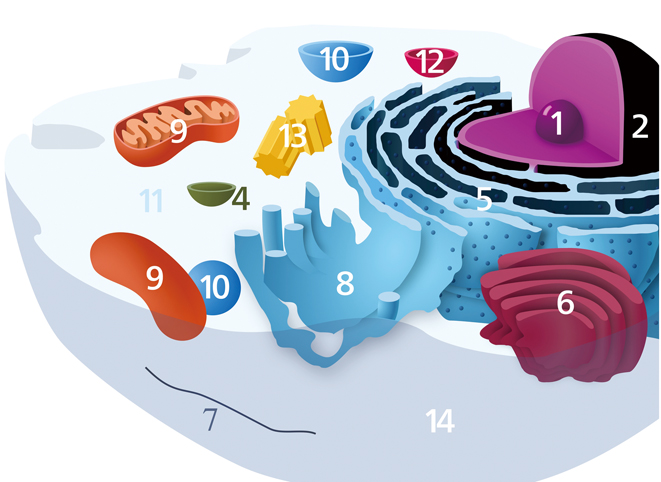
Illustrated here are the components of a typical animal cell, in which the nucleolus is situated at the center of the nucleus: 1, Nucleolus; 2, Nucleus; 3, Ribosome (little dots); 4, Vesicle; 5, Rough endoplasmic reticulum; 6, Golgi apparatus (or “Golgi body”); 7, Cytoskeleton; 8, Smooth endoplasmic reticulum; 9, Mitochondrion; 10, Vacuole; 11, Cytosol (fluid that contains organelles, comprising the cytoplasm); 12, Lysosome; 13, Centrosome; 14, Cell membrane. (Image by Kelvinsong - Own work, CC0, https://commons.wikimedia.org/w/index.php?curid=22952603)
Moreover, they observed that overexpression of wild type SLERT enhanced pre-rRNA expression. On the other hand, the decreased level of pre-rRNAs in SLERT knockout cells can be recovered by introducing wild type SLERT, but not egfp-SLERT, a mutant whose non-snoRNA sequence was replaced with a fragment of the non-relevant egfp sequence. All these observations indicated that the internal region of SLERT is essential for promoting rRNA transcription, and SLERT is capable of interfering with Pol I transcription via its non-snoRNA sequence.
How does SLERT regulate pre-rRNA transcription? To answer this question, they embarked on further experiments.
The Siege
In pursuit of the underlying mechanism of SLERT, the team made efforts to identify proteins closely associated with SLERT. They singled out the DDX21, a DEAD-box RNA helicase, and an abundant nucleolar protein. Via super-resolution imaging (SIM) and a series of biochemistry assays, they found that this RNA helicase turned hostile to pre-rRNAs in SLERT-knockout cells independent on its helicase activity.
The DDX21, in absence of SLERT, mistakenly kidnaped Pol I molecules to form a complex in the nucleolus. In the end, it formed a thick donut-like ring surrounding multiple Pol I molecules, clogging the pathway through which the rDNA binds to Pol I, and hence stopping the latter to accomplish the transformation from rDNAs to rRNAs.
Interestingly, fluorescence intensity of the pre-rRNA coating on the DDX21 ring indicated a positive correlation between the size of the ring and the concentration of pre-rRNA: the bigger the ring, the higher the level of pre-rRNA transcription in the nucleolus. This, the researchers inferred, suggested a positive correlation between the size of the DDX21 ring and the activity of Pol I. However, paradoxically, the DDX21 ring in whole actually suppressed Pol I transcription. Why?
Seems that the answer lies in the interaction of SLERT with DDX21. What is the magic of SLERT?
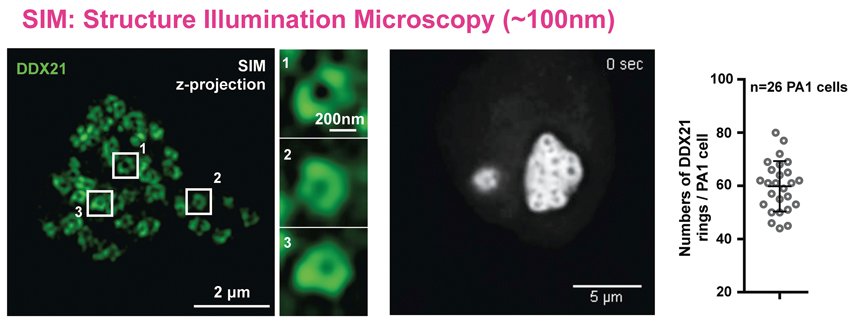
Structure illumination microscopy (SIM) observation shows that DDX21 molecules form some ring-like structures in the nucleolus. (Image by CHEN LL)
The Rescue
The researchers applied SIM again to study the spatial localization of SLERT, DDX21 and Pol I. They observed that SLERT was located to DDX21 rings rather than Pol I in wild type cells, and found almost no co-localization between SLERT and Pol I. A possibility might be, they inferred, SLERT could alter the conformation of DDX21 rings capping the Pol I complexes, thereby resulting in enhanced pre-rRNA production. Comparison between DDX21 rings in SLERT -depleted and -overexpressed cells indicated a positive regulation of the size of DDX21 rings by SLERT; and this further inspired the researchers of a possibility: The binding of SLERT to DDX21 might change the allosterical characteristics of the DDX21 molecules, hence promoting Pol I transcription.
To verify this speculation, the researchers applied a F?rster resonance energy transfer (FRET) assay to examine the conformational change of individual DDX21 molecules both in the presence and absence of SLERT. Their hypothesis was verified. Eventually, and with lines of evidence obtained from other molecular biological assays, they concluded that binding by SLERT allosterically alters individual DDX21 molecules, loosens the DDX21 ring, and liberates Pol I molecules from suppression by DDX21 – this ultimately fuels the rRNA transcription with sufficient materials and promotes the production of rRNAs.
However, devil is the other side of this angel. Over transcription of rRNA could invite disasters. Evidence shows that uncontrolled rRNA transcription is associated with tumorigenesis – SLERT can also promote cancer cell proliferation. As noted at the beginning of the story, accurately modulated pre-rRNA transcription is of vital importance in securing appropriate cell growth and development. Given that DDX21 itself suppresses whereas SLERT promotes pre-rRNA transcription, either SLERT expression or the coupled SLERT-DDX21 regulation could provide a promising target for anti-cancer therapies. Modulating the interaction between the two can achieve a balanced expression of pre-rRNAs and hence wholesome cell growth, the researcher speculated.
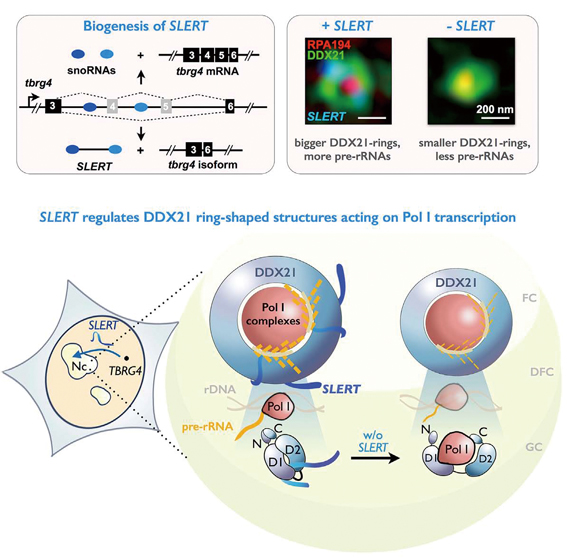
The graphical illustration shows how SLERT regulates rRNA transcription in human nucleolus. (Image by CHEN LL)

