By YAN Fusheng
In 1891, a New York surgeon named William Coley injected cancer patients with live bacteria, observing with fascination as some tumors shrank amid raging fevers. His crude experiments—later deemed reckless—nonetheless revealed a tantalizing truth: The immune system, when properly provoked, could attack cancer. Over a century later, researchers have transformed this observation into a precision strike force.
Published in Cell on March 3, a breakthrough study co-led by Dr. LIU Chenli of the Shenzhen Institutes of Advanced Technology (SIAT) and Dr. XIAO Yichuan of the Shanghai Institute of Nutrition and Health (SINH)—both affiliated with the Chinese Academy of Sciences (CAS)—reveals how genetically engineered Salmonella bacteria can selectively target tumors with high precision and efficacy. By reprogramming these microbes, the research team unraveled the mechanisms by which the bacteria infiltrate the complex tumor microenvironment (TME), skillfully bypassing the host’s immune defenses while activating robust anti-tumor effect.
DB1: A Precisely Designed Bacteria
The team created an engineered Salmonella strain, dubbed Designer Bacteria 1 (DB1), by stripping of its disease-causing genes and reprogramming with synthetic biology tools. Its mission: infiltrate tumors, survive their hostile environment, and ignite an immune revolt.
DB1 was equipped with an oxygen-sensitive “suicide switch”. So, DB1 self-destructs in healthy tissues (rich in oxygen) but tends to thrive in tumors’ oxygen-starved cores. DB1 also carries a secret weapon, listeriolysin O (LLO), a “molecular drill” originally evolved by the infamous Listeria monocytogenes (a foodborne pathogen) to pierce cell membranes. This borrowed toxin punches holes in cancer cells, letting DB1 spread like wildfire through tumors.
In mouse studies, DB1 therapy reduced bladder, skin, and colon tumors to scar tissue within weeks. The real magic lies in how it manipulates the tumor’s ecosystem—a discovery involving cellular “memory,” betrayed neutrophils, and a misunderstood immune molecule.
IL-10: The Builder of Cellular Memory
At the heart of DB1’s tumor-shrinking magic lies a molecular betrayal—the renegade behavior of Interleukin-10 (IL-10). This cytokine, long dismissed as a tumor collaborator for its role in exhausting cancer-fighting CD8+ T cells, now emerges as the vital player in DB1’s “tumor-targeting” and “tumor-clearing” effect. The key? Its paradoxical partnership with IL-10 receptor (IL-10R) and something never seen—a cellular memory forged by cytokine surge during early tumor growth.
When analyzing human tumors, researchers made a startling discovery—IL-10Rhigh immune cells (bearing abnormally high receptor levels) exist across most cancer types.
But why? The answer lies in hysteresis—imagine a thermostat that keeps your house warm long after you turn down the heat; that’s hysteresis—a system’s memory of past conditions. Tumor immune cells exposed to early IL-10 surges during early tumor growth developed a molecular “post-traumatic stress disorder”: their IL-10 receptors remained hypersensitive (IL-10Rhigh) even as cytokine tides receded. This created a biological paradox: a population simultaneously hyper-vigilant (receptors screaming at whispers) and functionally exhausted (incapable of mounting coherent attacks)—a self-sabotaging landscape where tumors hijack the immune system’s alarm bells to fortify their own citadels—or, in another name, the notorious tumor microenvironment (TME).
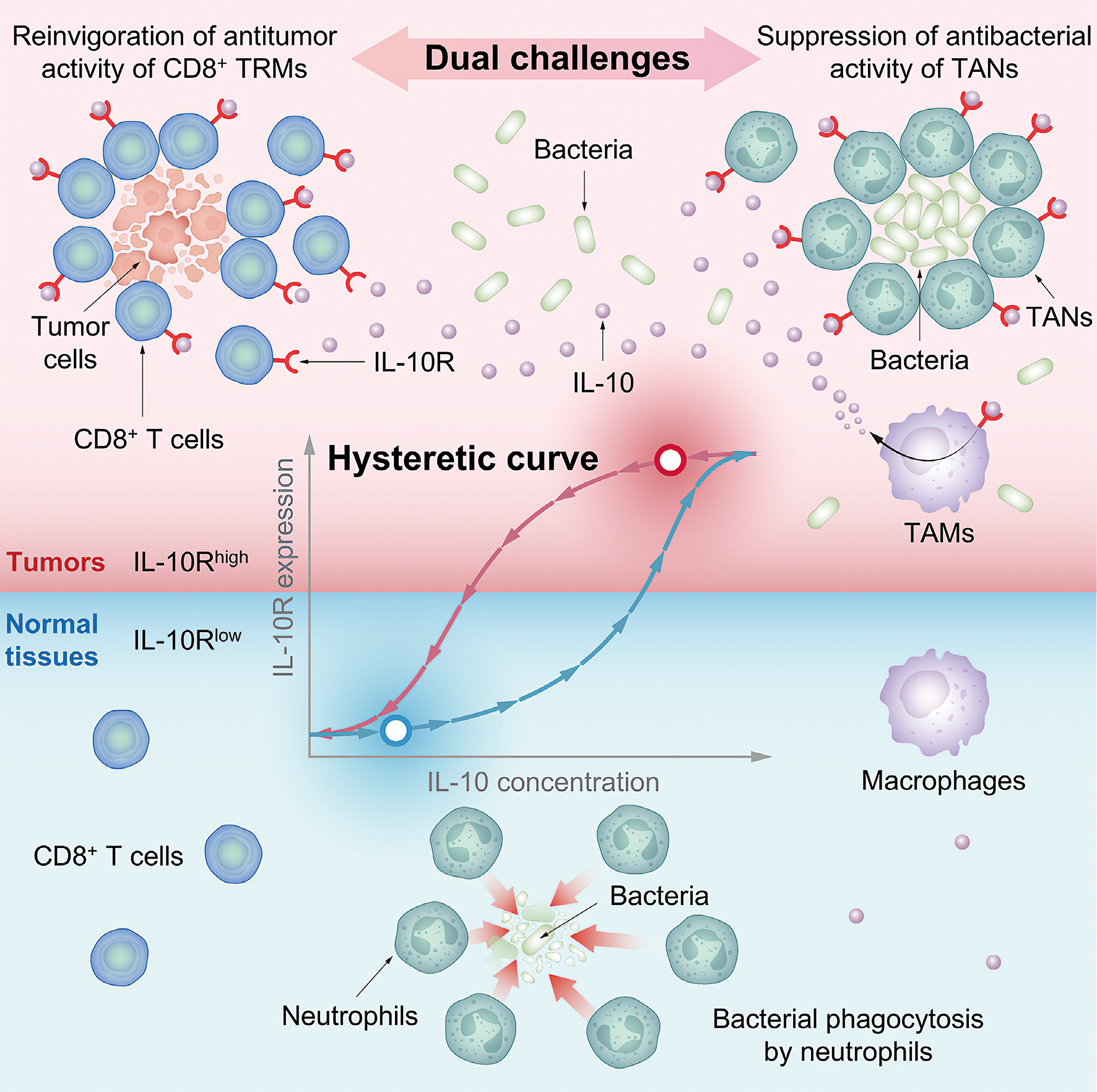
Leveraging the IL-10Rhigh state of the immune cells, a “memory” of IL-10 cytokine surge during early tumor formation, an engineered Salmonella enterica strain can reconcile the longstanding dual challenge in bacterial cancer therapy: evading antimicrobial immune defenses while promoting antitumor activity. (Graphic: CAS)
The Memory Hijack
Now the stage is set, and the time comes for DB1’s genius stunts. Like a hacker exploiting cached passwords, the engineered bacteria operate like a master spy, hijack this “memory” to its own benefit to evade anti-bacterial immunity.
DB1 coerces tumor-associated macrophages (TAMs) into flooding the tumor with IL-10 (10x surge in 24h). The enhanced level of IL-10 efficiently paralyzes tumor-associated neutrophils (TANs)—the very cleaners meant to destroy bacteria. Paralyzed TANs form hollow defense rings (see graphic), creating safe zones where DB1 thrives unchallenged.
In a stunning reversal, IL-10 binds hyper-sensitive receptors on “exhausted” CD8+ T cells—the immune system’s forgotten assassins. Like waking sleepers with adrenaline, it restores their tumor-killing fury.
The final act plays out with strategic precision—the engineered bacterial agents infiltrate tumors, survive their hostile environment, and ignite an immune revolt.
The result in mice turns out to be very promising. After intravenous injection, DB1 cells deliver 90% reduction in melanoma growth, 100% survival vs. rapid deaths in controls, and immunity to tumor rechallenge (like a cancer vaccine). Like it says in the article, DB1 induced an antigen-specific immune memory in the treated mice, preventing tumor recurrence and metastasis.
This is no simple drug delivery. It’s ecological warfare—exploiting evolved tumor defenses to create a self-destruct sequence.
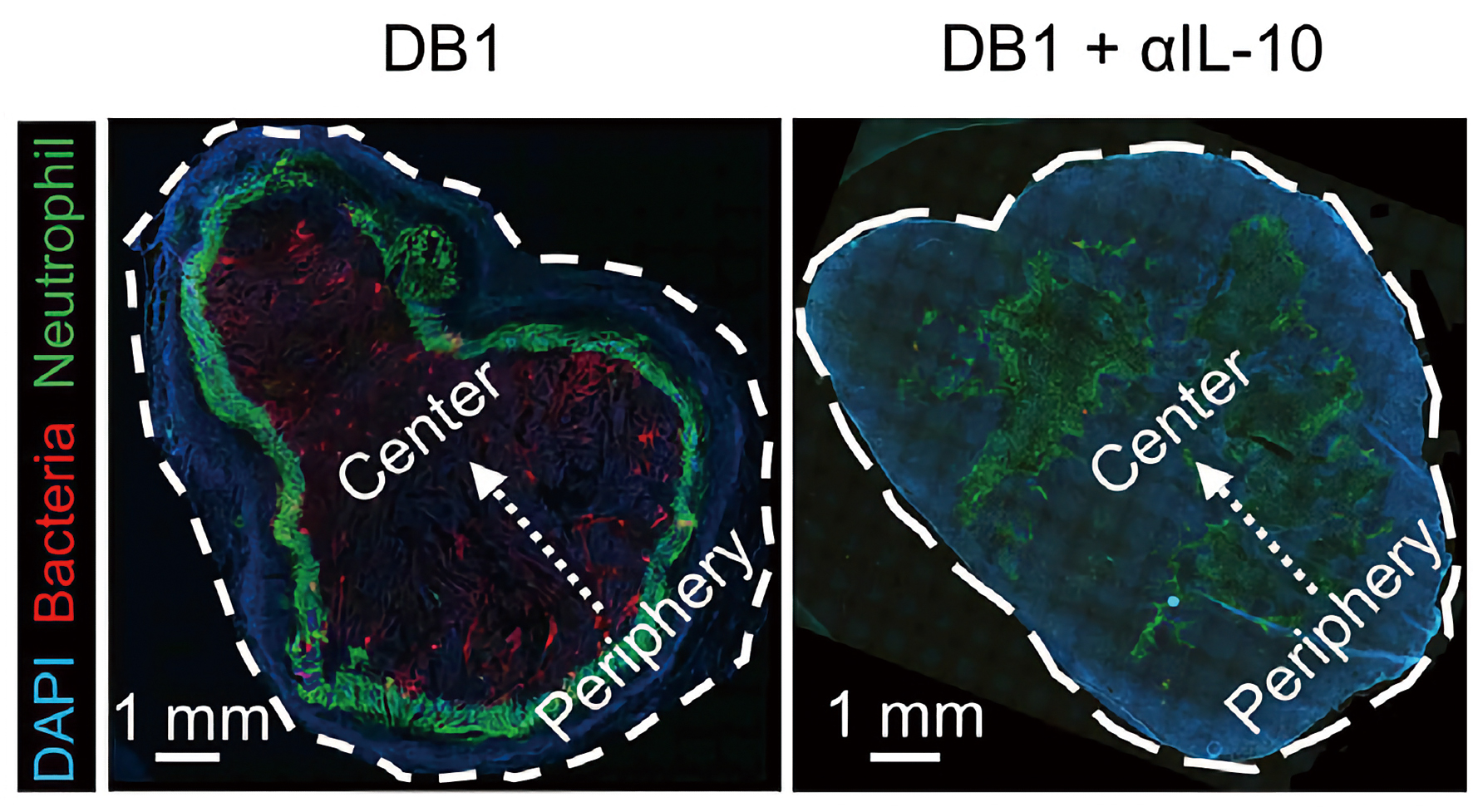
Left panel shows this biological siege: green TANs immobilized at periphery (failed defenders), red DB1 colonies feasting in the core. Right panel shows neutrophils storm the bacterial stronghold and wipe them out when IL-10 is blocked.(Graphic: CAS)
Bacterial Cancer Therapy: A Paradigm Shift
Previous attempts to harness IL-10’s therapeutic potential stumbled in clinical trials—direct cytokine injections failed to concentrate adequately in tumors, undermining efficacy. The new method breaks the spell. As demonstrated in the team’s Cell paper, DB1-associated therapeutics can trigger persistent IL-10 expression in tumor locally for nearly 14 days.
DB1 doesn’t just deliver IL-10 better. It redefines cytokine delivery as a time-released, space-constrained, self-amplifying siege. By confining cytokine activity to malignant tissue, it sidesteps off-target toxicity while maintaining therapeutic levels where they matter most—a paradigm-shifting strategy that could finally unlock IL-10’s full anticancer potential.
In summary, DB1’s story mirrors classic espionage: a double agent (bacteria) infiltrating enemy lines (tumors), turning their weapons (IL-10) against them. Unlike Coley’s crude toxins, this approach is exquisitely precise—a testament to how far bacterial cancer immunotherapy has come.
Reference
Chang, Z., Guo, X., Li, X., et al. (2025) Bacterial immunotherapy leveraging IL-10R hysteresis for both phagocytosis evasion and tumor immunity revitalization. Cell. 188(7):1842–1857.e20. doi:10.1016/j.cell.2025.02.002

