By YAN Fusheng
For years, scientists believed that astrocytes, the star-shaped brain cells, only had two options—become helpful protectors that shield the brain or become harmful destroyers that damage brain tissue. But a new discovery shows this isn’t the full story.
In the intricate tapestry of the brain, astrocytes—once considered mere support cells—have emerged as dynamic players in the drama of neurodegenerative diseases. A recent study published in Nature Aging on January 8 has unveiled an interesting discovery: astrocytes don’t just switch into “protective” or “toxic” states but undergo an unexpected transition—the neuroprotective state during neuroinflammation is transient, unstable, and prone to flipping into a neurotoxic state over time.
The Astrocyte Dance: From Protector to Perpetrator
Astrocytes, the star-shaped cells that make up a significant portion of the brain’s glial cell population, have traditionally been categorized into two camps: neurotoxic and neuroprotective. However, this binary view is proving to be too simplistic. The new research, led by Dr. ZHOU Haibo and Dr. ZHANG Liansheng at the Institute of Neuroscience (ION), under the Chinese Academy of Sciences, suggests that astrocytes don’t just flip into one state or another but instead transition through intermediate stages. The neuroprotective substate represents an unstable and intermediate state that is induced temporally during the induction of nonreactive-to-neurotoxic transition.
Using time-series multiomic sequencing, the team looked at gene expression and other molecular markers over time, allowing them to see the changes happening step by step, rather than just comparing two static states. The team observed that substate transitions of astrocytes occurred at both mRNA and protein levels.
Imagine astrocytes as dancers on a neuronal stage. Initially, they perform a protective ballet, secreting neurotrophic factors that nurture neurons. But under the stress of neuroinflammation, they begin to change their dance style, eventually adopting a toxic tango that can harm the very neurons they once protected. This transition is not random; it’s a carefully choreographed sequence regulated by the mTOR signaling pathway.
The mTOR Conductor
The mTOR pathway, a master regulator of cell growth and metabolism, appears to be the conductor of this cellular dance. When astrocytes are exposed to inflammatory signals like IL-1α, TNF, and C1q, the mTOR pathway is dampened. This suppression allows the astrocytes to enter a neuroprotective phase, where they secrete beneficial factors. However, if the inflammation persists, the mTOR pathway remains suppressed, pushing the astrocytes into a neurotoxic state.
The researchers found that by modulating the mTOR pathway, they could influence the behavior of astrocytes. Using rapamycin, an mTOR inhibitor, they were able to mitigate the neurotoxic effects of astrocytes in mouse models of Alzheimer’s and Parkinson’s diseases. This suggests that targeting mTOR in astrocytes could be a possible therapeutic strategy for neurodegenerative diseases.
Since rapamycin lacks precision in targeting astrocytes and risks off-target effects, researchers tested whether directly blocking the mTOR protein in astrocytes could protect neurons. Using a gene therapy tool to silence mTOR in these cells, they reduced toxic signals and prevented dopamine neuron death in a Parkinson’s disease model—without disrupting protective functions—highlighting a safer, targeted approach for brain diseases.
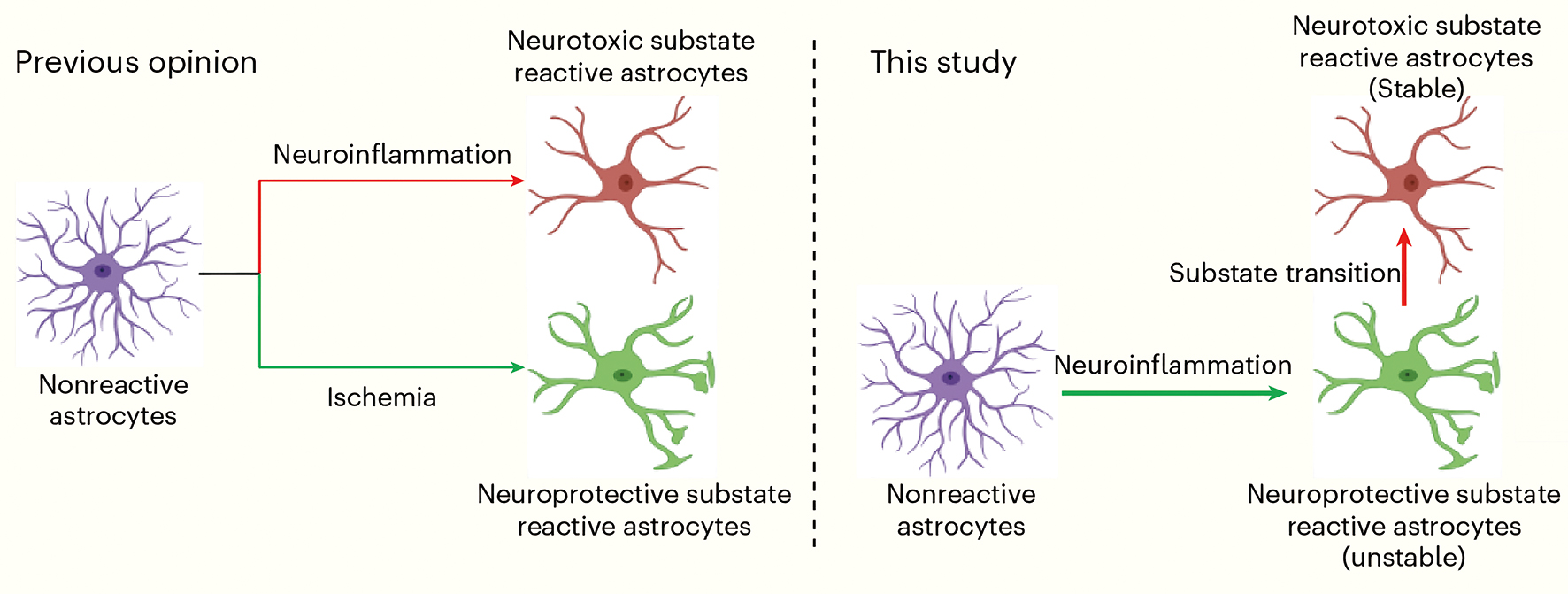
New study reveals astrocytes don’t just flip into “toxic” or “protective” states (left) but shift fluidly through dynamic states (right)—overturning the rigid binary view of their role in brain health. (Graphic: ION)
The Balance of Power
The study also revealed an imbalance in astrocyte substates in both mouse models of neurodegenerative diseases and in human patients. In Alzheimer’s disease, for example, there is a shift towards a higher proportion of neurotoxic astrocytes and a decrease in neuroprotective ones. This imbalance correlates with disease progression and even occurs in normal aging, albeit to a lesser extent.
Think of this imbalance as a seesaw tilting towards toxicity. In healthy brains, the seesaw is balanced, with astrocytes oscillating between protective and reactive states as needed. But in diseased brains, the scale tips, leading to sustained neurotoxicity and neuronal damage.
New Avenue for Treatment
The implications of this research are profound. If astrocytes can be coaxed to remain in their neuroprotective state, or if their transition to a neurotoxic state can be halted, it could pave the way for new treatments that protect neurons from degeneration.
This study not only deepens our understanding of astrocyte biology but also opens new avenues for therapeutic intervention in neurodegenerative diseases. As researchers continue to unravel the complexities of these enigmatic cells, the hope is that we can one day harness their power to preserve neuronal health and combat the devastating effects of diseases like Alzheimer’s and Parkinson’s.
Reference
Zhang, L., Xu, Z., Jia, Z., et al. (2025) Modulating mTOR-dependent astrocyte substate transitions to alleviate neurodegeneration. Nature Aging. 5(3), 468–485. doi:10.1038/s43587-024-00792-z

