By YAN Fusheng (Staff Reporter)
“It is not birth, marriage, or death, but gastrulation, which is truly the most important time in your life,” said Lewis Wolpert in 1986, an eminent developmental biologist. Because during gastrulation, cell migrations lead to a massive reorganization of the embryo from a simple spherical ball of cells, the blastula, into a multi-layered organism. A dearth of primate embryo samples at the gastrulation stage has, however, limited our understanding of this critical event in primates. Now, a newly established platform to grow monkey embryos in dishes could be the ice-breaker.

Left: One representative d.p.f. 14 IVC embryo stained with antibodies for OCT4 (green) and GATA6 (red). Arrowheads indicate presumptive gastrulating cells. Scale bar, 60 mm. Right: Diagram based on the left image. (Credit: Science)
It is an epic journey for a single fertilized egg to grow into a healthy baby, in which the on and off of particular genes and the whereabouts of particular cells are so dynamic, but yet orchestrated. How the cells decide to switch their genes and make movements remains to be fully understood, particularly during gastrulation – an early embryonic stage involved with massive cell migrations to form the three germ layers that later give rise to all the different organs and tissues.
For human embryos, gastrulation begins roughly at 14 days after fertilization. Due to ethical concerns, the culturing of human embryos for research uses beyond this stage is not supposed, because gastrulation is considered the time point for one to think that this ball of cells is actually growing into a living creature.
However, to illustrate the roadmap of human embryo development, scientists must get to fully understand the gastrulation process. An alternative option is to work on other primate embryo model that is very similar to the human embryo.
Besides, it would help a lot if these embryo samples could be cultured in vitro instead of growing in wombs, so that they could be observed and investigated in real time.
To address that, CAS scientists developed a culture system that supports the growth and development of cynomolgus monkey embryos for up to 20 days after fertilization and beyond gastrulation. Instead of growing embryos in surrogate wombs, they cultured these primate embryo samples in petri dishes.
“It is very exciting and I am delighted that our method for culturing human embryos was useful here and can be adopted further,” said Dr. Magdalena Zernicka-Goetz, a professor at the University of Cambridge who has pioneered in culturing embryonic cells in a dish, “Perhaps the most surprising finding for me is that the authors were able to culture the embryos up to day 20 (which is beyond gastrulation). It’s a true achievement as now there is a system to study gastrulation in vitro in a model very similar to the human embryo.”
This progress was made by a joint team led by Dr. WANG Hongmei and Dr. LI Lei from the CAS Institute of Zoology (IOZ), and Dr. ZHENG Ping from the CAS Kunming Institute of Zoology (KIZ), and appeared as a research article in Science on October 31.
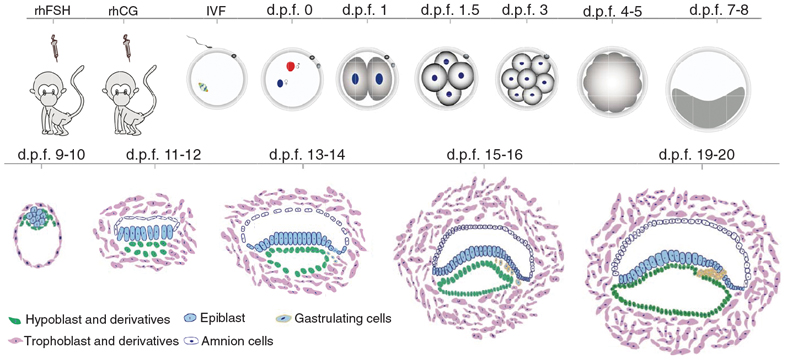
Establishment of the IVC system for monkey embryos and the time scheme of superovulation by injecting two female hormones, in vitro fertilization (IVF), and one-cell to gastrulation transition in the IVC system. At d.p.f. 13-14, gastrulating cells are on the move and at d.p.f. 19-20, the three layers are formed that later grow into different organs and tissues. The d.p.f. stands for days post fertilization (Credit: Science)
To fulfill this accomplishment, the authors first sought to optimize an IVC system for the culture of mouse embryos, which was established previously. Then, they used this optimized system to culture cynomolgus monkey embryos to 20 days. They also performed whole-mount embryo immunostaining and reverse transcription PCR – techniques to reveal the whereabouts and abundance of proteins and nucleic acids (the DNA and RNA molecules) – to gain molecular landscapes along embryonic development, particularly the gastrulation. The results demonstrated that the IVC embryos recapitulated the key events of in vivo early embryo development, including segregation of the epiblast and hypoblast, formation of the amniotic and yolk sac cavities, appearance of the primordial germ cells and the initiation of gastrulation.
Single-cell RNA-seq analyses – a technique to tell cellular differences by sequencing RNA from individual cells, and so as to define cell types – showed that the IVC embryos have cell types with gene signatures that are very similar to their in vivo counterparts harvested from wombs. This means that these IVC embryos could be used as valuable and reliable models to study primate early embryonic development and particularly the gastrulation process.

Monkey embryos grow in vitro beyond early gastrulation. The in vitro cultured embryos were stained with the antibodies for OCT4 (green, a major hallmark for epiblast cells) and GATA6 (red, a major hallmark for hypoblast cells), and hematoxylin and eosin staining. Single-cell transcriptome analysis revealed the similarities among cell types and developmental trajectory of epiblast derivatives in the in vitro and in vivo monkey early embryos. (Credit: Science)
Altogether, this study provides compelling evidence supporting that the cynomolgus monkey embryos can develop beyond early gastrulation and up to 20 days post fertilization in their IVC system. Furthermore, this study provides novel information on cell lineage specification during primate early post-implantation development.
More importantly, in combination with CRISPR-Cas9-mediated gene editing and cell lineage tracing, this system could accelerate our understanding of the mysterious dynamics of early embryonic development in primates, with possible relevance to human early embryonic development and diseases, such as abnormal pregnancy and fetal abnormalities.
“This method opens a completely new avenue to investigate primate gastrulation in real time. Combined with the power of modern microscopy techniques, this reported system will enable the discovery of key processes underlying primate gastrulation, which cannot be easily captured using fixed specimens or analyzing other non-primate species,” said Dr. Nicolas Plachta, a National University of Singapore professor specialized in imaging how cells decide their fate, shape and position in the living mammalian embryo.
References
Huaixiao Ma, Jinglei Zhai, Haifeng Wan, Xiangxiang Jiang, Xiaoxiao Wang, Lin Wang, Yunlong Xiang, Xiechao He, Zhen-Ao Zhao, Bo Zhao, Ping Zheng*, Lei Li*, Hongmei Wang*, (2019) In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science (New York, N.Y.) 366, eaax7890. doi: 10.1126/science.aax7890.
I. Bedzhov, M. Zernicka-Goetz*, (2014) Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 156, 1032. doi: 10.1016/j.cell.2014.01.023.
A. Deglincerti, G. F. Croft, L. N. Pietila, M. Zernicka-Goetz, E. D. Siggia, A. H. Brivanlou*, (2016) Self-organization of the in vitro attached human embryo. Nature 533, 251. doi: 10.1038/nature17948.
T. Nakamura, I. Okamoto, K. Sasaki, Y. Yabuta, C. Iwatani, H. Tsuchiya, Y. Seita, S. Nakamura, T. Yamamoto, M. Saitou*, (2016) A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 537, 57. doi: 10.1038/nature19096.

