By YAN Fusheng (Staff Reporter)
Baculoviruses infect insects in nature by targeting their larvae. They can also pass on genes into mammalian cells (including human cells) without producing progeny viruses via a process termed transduction. For that, they are promising vectors for human gene therapy. There is, however, an unsolved obstacle that the mammalian transduction is very inefficient. To address that, scientists from the CAS Wuhan Institute of Virology (WHIOV) sought to figure out the major roadblock to mammalian cell entry of baculovirus and find out a solution to overcome it.
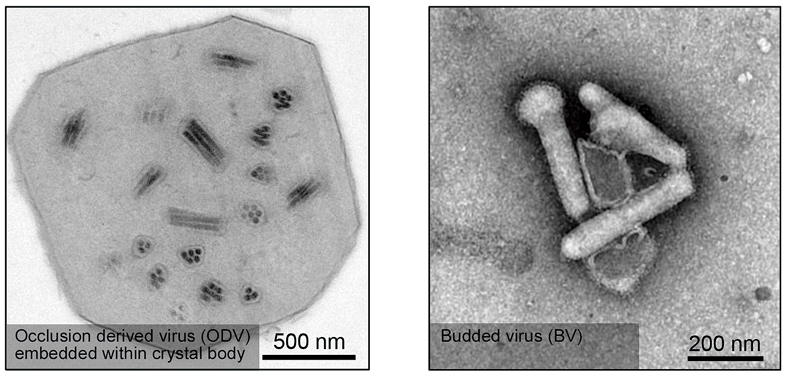
Baculovirus evolved into two different forms (shown by TEM imaging), occlusion-derived virus (ODV) and budded virus (BV), in which ODV transmits the viruses between different larva individuals while BV mediates cell-to-cell transmission. Over the past few years, baculovirus has been recognized as a promising vector for human gene delivery and therapy. (Credit: WANG Manli, WHIOV)
Baculoviruses are large, rod-shape DNA viruses that specifically infect insects, mainly the larvae of butterflies and moths. Though they do not pose a threat to public health, baculoviruses are quite interesting. After millions of years of co-evolution with their insect hosts, baculoviruses have developed unique replication cycles featuring with two distinct virus forms: occlusion-derived virus (ODV) and budded virus (BV).
The ODV form usually consists of one to multiple nucleocapsids (a viral genome coated by capsid proteins) embedded within a certain crystal body. This unique form allows the virus particles to survive outside of a natural host before they can infect the next that accidentally engulfs it. Actually, ODVs have been commercialized as biopesticides for insect control. The BV form of baculoviruses consists of a single nucleocapsid enveloped in a host-derived membrane that is in nature a sprout of membrane taken by a budding nucleocapsid. At its outer envelope, there exist abundantly virally encoded membrane fusion proteins that play key roles in virus cell entry. BVs spread infection within larval body via cell-to-cell transmission. Unlike ODVs that need to be eaten by a host to initiate infection, BVs can directly infect cultured insect cells in vitro. Hence, BVs carrying external genes represent valuable tools to produce recombinant proteins from insect cells.
More interestingly, although baculoviruses infect insects in nature, they also transduce a wide variety of mammalian cells (including human cells). Unlike virus infection, virus transduction does not produce progeny viral particles within host cells and does not involve virus replication. But baculoviruses can enter into mammalian cells and allow the carried external genes (with a considerable capacity) to be expressed. This makes baculovirus a promising tool for human gene delivery and gene therapy. More importantly, it skirts the risks caused by viral genome integration – a safety concern by using other virus-based gene therapy, such as lentivirus and adenovirus that insert their viral genomes into the host. However, the major problem is that baculoviruses transduce mammalian cells at a very limited efficiency. So, it is required to figure out what goes wrong or what is the roadblock to mammalian cell entry of baculoviruses.
By comparing the mechanisms between insect and mammalian cell entry, research groups led by Prof. WANG Manli and Prof. WANG Hualin from the CAS Wuhan Institute of Virology (WHIOV) unraveled the major roadblock to mammalian cell entry of baculovirus, and provided a solution to overcome this obstacle. Their study was published in Journal of Virology on July 17.
To sort out why baculovirus underperforms on entering mammalian cells, researchers performed a detailed comparative study on entry mechanisms of the prototypical baculovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV) into insect and mammalian cells. They chose AcMNPV because of its popularity in the science community of studying baculoviruses, as well as its sequenced genome. More importantly, AcMNPV has been considered an ideal vector for gene delivery and therapy in the past decades. It seems one of the major obstacles that prevents AcMNPV from being used in practice is its low efficiency of mammalian cell entry.
A successful cell entry of most viruses usually includes internalization via endocytosis – an inward-budding membrane process that engulfs molecules or particles into cells, endosomal escape, nucleocaspid disassemble and genome release.
They found that AcMNPV can be efficiently internalized into endosomes, membrane-bound vesicles pinched off from the inward-budding membrane during endocytosis. But this internalizing process is somewhat like the cell taking a virus-containing “capsule”. Getting inside endosomes alone does not guarantee a successful cell entry. Apparently, these capsulated viruses need to find a way to get out and go to some place where they can somehow disassemble themselves and hijack the host’s molecular machineries to complete their replication cycle. Otherwise, once the endosomes turn into lysosomes as time goes by, the trapped viruses would be destroyed. So, it seems that a timely escape from endosomes is critical for a successful cell entry.
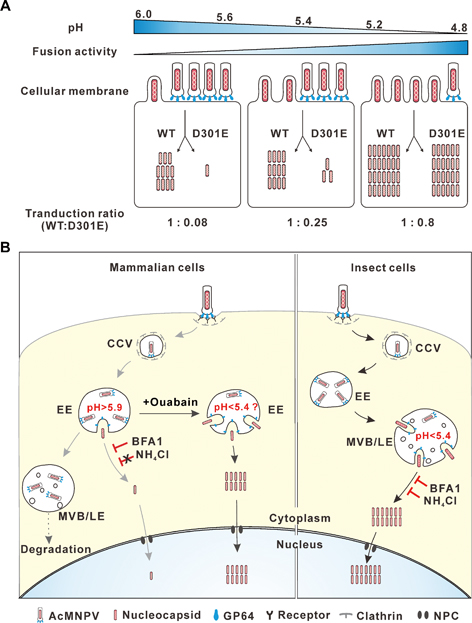
A proposed mechanism of mammalian and insect cell entry of baculovirus AcMNPV. In mammalian cells, the AcMNPV viruses are taken inside and transported to the early endosomes (EEs), where very few virions (here a virion of AcMNPV contains a nucleocapsid and a lipid envelope) can fuse with the membrane and release the nucleocapsids into the cytoplasm. Most of them are trapped and then degraded within later endosomes (LEs) replete with destructive machineries. However, this situation could be rescued by using Ouabain, a small cardiac steroid molecule that specifically acts to decrease the pH of EEs and greatly enhance viral fusion. In insect cells, the taken-in viruses escape from the acidic LEs quite well due to the efficient membrane fusion between the viruses and the endosomal membrane. In both cell types, released nucleocapsids are transported to the nucleus where they somehow disassemble themselves and release the contained viral genomes. (Credit: WANG Manli, WHIOV)
Indeed, researchers found that membrane fusion – an critical event in cell entry that involves the outer envelope of viruses “kissing” with the inner membrane of endosomes, triggering the escape of nucleocapsids from endosomes into cytoplasm – in mammalian cell entry of AcMNPV is less efficient compared to that in insect cell entry. By measuring the endosomal pH, they found that pH values in the early endosomes (EEs) where AcMNPV fused from mammalian cells were relatively higher than that from insect cells. Considering that lower pH can better activate the membrane fusion protein GP64 at the outer envelope of AcMNPV, the relatively high pH in mammalian EEs inhibits membrane fusion and nucleocapsid escape. The pH-dependent activity of membrane fusion protein seems critical to ensure a timely escape of baculovirus from the endosomes that later could turn into destructive lysosomes. Actually, this sort of “pH-sensing” mechanism is commonly shared among viruses, which is somewhat self-evident that a drop of pH is usually a reliable cue for viruses that they have already been taken inside.
They also found that mutants of the major viral fusion protein GP64 with decreased potency to facilitate membrane fusion did not affect virus infection of insect cells, whereas virus transduction of mammalian cells was severely impaired. This suggests that mammalian cell entry of AcMNPV is more stringently dependent on the fusion potency of GP64. More importantly, they prescribed a remedy for this dilemma. They found that treatment with Ouabain, a small cardiac steroid molecule that specifically acts to decrease the pH of EEs, greatly enhanced viral fusion with endosomes, leading to an improved mammalian cell entry of baculovirus. Based on these findings, they proposed a mechanism of mammalian and insect cell entry of baculovirus, as well as a solution to improve mammalian cell entry.
Overall, they illustrated that virus membrane fusion in the early endosomes is the major obstacle for efficient mammalian cell entry of baculovirus. More importantly, they also provided a novel strategy of enhancing membrane fusion by decreasing endosomal pH to improve baculovirus-based gene delivery or therapy.
Reference
L. Hu, Y. Li, Y. J. Ning, F. Deng, J. M. Vlak, Z. Hu, H. Wang*, M. Wang*, (2019) The major hurdle for effective baculovirus transduction into mammalian cells is passing early endosomes. Journal of Virology 93. doi: 10.1128/jvi.00709-19.

