Reported by YAN Fusheng
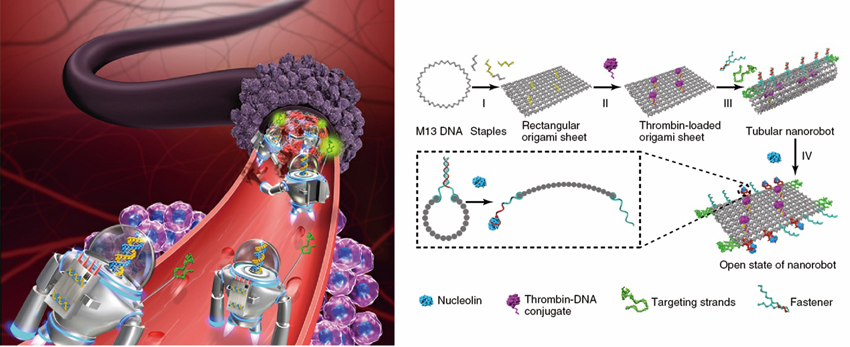
Left: Illustration of the circulating DNA nanorobots working on site by forming blood clots to cut the supplies for tumor cells. Right: Design of the DNA Nanorobot with thrombin in pink, nucleolin in blue and navigating DNA aptamers in green. One of the fasteners that unfold the nanorobot upon docking onto nucleolins is zoomed in. (Credit: NCNST)
Scientists have been long seeking to use smart nanorobots to spontaneously spot and cure human diseases. In February 2018, a joint team devised a kind of DNA-based nanorobots for cancer therapy, led by Profs. NIE Guangjun, DING Baoquan and ZHAO Yuliang from the CAS National Center for Nanoscience and Technology (NCNST) based in Beijing, China and Prof. YAN Hao from the Arizona State University of USA. Once introduced into the circulation, these nanorobots can trigger the clotting inside blood vessels at the tumor site, to cut supplies to tumor cells, and starve them to death.
These nanorobots consist of three functional parts: DNA origami, thrombin and DNA aptamer (see figure Right). DNA origami is a kind of precise nanoscale shape or pattern, folded from a long single-stranded scaffold DNA (M13 DNA in this case) and hundreds of short helper DNA strands (the staples). In this design, DNA origami, in the shape of a rectangular raft, is loaded with thrombin, an enzyme that causes blood clotting. DNA aptamer is a short single-stranded DNA that can bind to a certain target. In this case, it binds to nucleolin, a tumor biomarker highly expressed within tumor environment. Two forms of DNA aptamers are used in the design, targeting strands and fasteners (figure Right). The targeting strands are anchored at the ends of tube for the navigation of nanorobots; The fasteners suture and lock the origami raft into a tube to form the smart nanorobots.
Highlighted in this design is some automatic mechanism inherent to the DNA functional parts that makes the nanorobots “smart”. The targeting strands allow the nanorobots better able to navigate into the blood vessels at the tumor site. Once the nanorobots enter into these tumor-associated vessels, they will unfold themselves upon the docking of nucleolins – a surface marker that highly expressed on the vessel walls – onto the fasteners. Upon unfolding, the loaded thrombins are spurred into work to build up blockage by catalyzing the formation of blood clots. However, when the nanorobots are in the close state, the thrombins are sealed within the origami tube, and thus are prevented from causing blood clots. This smart feature of responsive unfolding ensure the clotting only occurs at the tumor site, not somewhere else that could damage normal tissues.
These DNA nanorobots have been demonstrated to be able to shrink various tumors in mice models, and safe to use in miniature pigs without eliciting undesired systematic blood clots and detrimental immune responses, confirmed the researchers.
Though its clinical relevance remains to be checked in human, this work sets a paradigm for designing smart nanorobots for tumor therapies. A setback is, however, the DNA strands used in the robots involve artificial synthesis and do not come cheap; while it takes a large number of DNA strands to build up one single nanorobot.
To advance DNA nanorobots for human use, scientists still need to figure out how to reduce the costs for both DNA synthesis and the production of DNA nanostructures with high purity, stated the researchers in another review paper.
Reference
Suping Li, Qiao Jiang, Shaoli Liu, Yinlong Zhang, Yanhua Tian, Chen Song, Jing Wang, Yiguo Zou, Gregory J. Anderson, Jing-Yan Han, Yung Chang, Yan Liu, Chen Zhang, Liang Chen, Guangbiao Zhou, Guangjun Nie*, Hao Yan*, Baoquan Ding*, Yuliang Zhao*, A DNA Nanorobot Functions as a Cancer Therapeutic in Response to a Molecular Trigger In Vivo. Nature biotechnology 36, 258 (Published: February 12, 2018). doi: 10.1038/nbt.4071

