Reported by YAN Fusheng
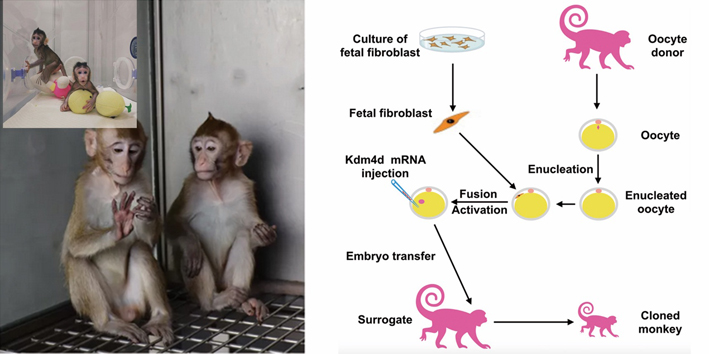
Left: The two cloned monkeys (“Zhongzhong” and “Huahua”) have grown up (inlet). Right: Schemed workflow for creating cloned monkeys by somatic cell nuclear transfer (SCNT) using fetal monkey fibroblasts as the nuclear donor. (Credit: ION)
The biological differences between the widely used rodent models and humans have caused many fiascos in pharmaceutical development. Drugs proven to be effective in rodents do not necessarily guarantee they work for humans. Unfortunately, this speaks for most cases. Thereby, animal models that mimic humans to the most are of great value and badly needed in biomedical studies and pharmaceutical tests. Non-human primates are considered to be the best animal models, given that they are genetically the closest to humans. Cloning these animals, however, is a longstanding challenge; many attempts ended with no reward.
Things changed early 2018. Scientists led by Profs. SUN Qiang and LIU Zhen at the CAS Institute of Neuroscience (ION)/CAS Center for Excellence in Brain Science and Intelligent Technology in Shanghai successfully cloned two live macaque monkeys,ZhongzhongandHuahua. These cloned monkeys and more to come with customized disease conditions could represent ideal models for studying human biology and diseases.
The team accomplished this feat via somatic cell nuclear transfer (SCNT), the same method that has made Dolly-the-sheep in the 1990s. This method replaces the nucleus of a chosen egg cell (the host) with the one extracted from a differentiated somatic cell (or body cell, the donor). After nuclear transfer, the donor cell nucleus is somehow “reprogrammed” by its host egg cell to regain the once lost ability called “totipotency,” the ability to differentiate into different types of body cells. Stimulated with a proper “shock,” the fused cell starts to divide and gradually forms an embryo that can be transplanted into a surrogate womb, where it can ultimately develop into a full-term fetus.
Previous attempts using adult somatic cells as nuclear donors failed to yield live monkey offspring. The poor reprogramming potency of the adult nuclei was considered to take the blame. To bypass this pitfall, the CAS team chose fetal nuclei as the DNA donor, given that they could be more readily to be reprogrammed by the host egg cell. Besides, by using fetal fibroblasts as donor cells coupled with in vitro screening, researchers can quickly obtain large amounts of identical donor nuclei that have the same gene edits. As a result, many genetically identical monkey models can be quickly produced to meet research needs.
The researchers also made other efforts to further improve the reprogramming potency of these transferred fetal nuclei.
In the fetal nuclei, exist some particular genomic regions called reprogramming-resistant regions (RRR) where the genomic DNAs are densely packed and are hard to be reprogrammed. These regions are featured with increased level of certain methyl tags on the histones, named histone 3 lysine 9 trimethylation (H3K9me3). To tackle this problem, the team managed to unlock these dense packages.
Minimal DNA packaging units start to form when genomic DNAs wrap around histones. Chemically tagging the core histones with methyl or acetyl can either tighten or loosen the DNA packaging, respectively. Therefore, less methylation or more acetylation on the histones can turn the chromosomes into a relatively relaxed state, whereby a chromosome is more accessible for the reprogramming process. For this sake, researchers injected H3K9me3 demethylase Kdm4d mRNA to greatly reduce these methyl tags at the one-cell stage. Meanwhile, they also treated the fused cell with histone deacetylase inhibitor trichostatin A to enhance the overall acetylation on the histones. With this combined treatment aimed at enhanced reprogramming potency of these transferred DNA, they achieved an improved success rate and quality of embryonic development.
Genetic analysis confirmed that the nuclear DNAs of the cloned monkeys was identical to the donor fetal fibroblasts, and the mitochondrial DNA was identical to the egg cell donors, indicating successful cloning of monkeys. The success of cloning macaques has broken the technical barriers, and created a new era, of using non-human primates as experimental models, a truly exciting milestone in biomedical studies.
“In near future, China may grow into a global hub for biomedical studies and pharmaceutical tests for human diseases,” says ION director Prof. POO Muming, who is a co-author of the study.
Reference
Zhen Liu, Yijun Cai, Yan Wang, Yanhong Nie, Chenchen Zhang, Yuting Xu, Xiaotong Zhang, Yong Lu, Zhanyang Wang, Muming Poo, Qiang Sun*, Cloning of Macaque Monkeys by Somatic Cell Nuclear Transfer. Cell 172, 881 (Published: January 24, 2018). doi: 10.1016/j.cell.2018.01.020

