Hydrogenation catalysts frequently impose a compromise between activity and selectivity, where maximizing one property inevitably diminishes the other. Researchers from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with scholars from University of Science and Technology of China and the Karlsruhe Institute of Technology in Germany, cracked this dilemma by engineering bimetallic catalysts with atomic precision—a breakthrough that boosts hydrogenation efficiency by 35-fold while maintaining pinpoint accuracy, resolving the stubborn activity-selectivity paradox.
Published in Chem on January 3, 2025, the study showcases platinum-iron clusters (Pt-Fe-Pt heterotrimers) where isolated Pt atoms bind to Fe nanoparticles. By precisely tuning their bonding structure, the team enabled these clusters to selectively hydrogenate crotonaldehyde’s C=O bond—producing crotyl alcohol with 99% preference over competing reactions. The atomic arrangement allows one Pt atom to anchor the substrate, while adjacent Fe and Pt sites activate and hydrogenate target bonds—a “lock-and-key” mechanism decoded at molecular resolution. This atomic-scale tailoring—combining nanocluster synthesis with surface alloying—sets a blueprint for next-generation catalysts, merging industrial efficiency with molecular precision.
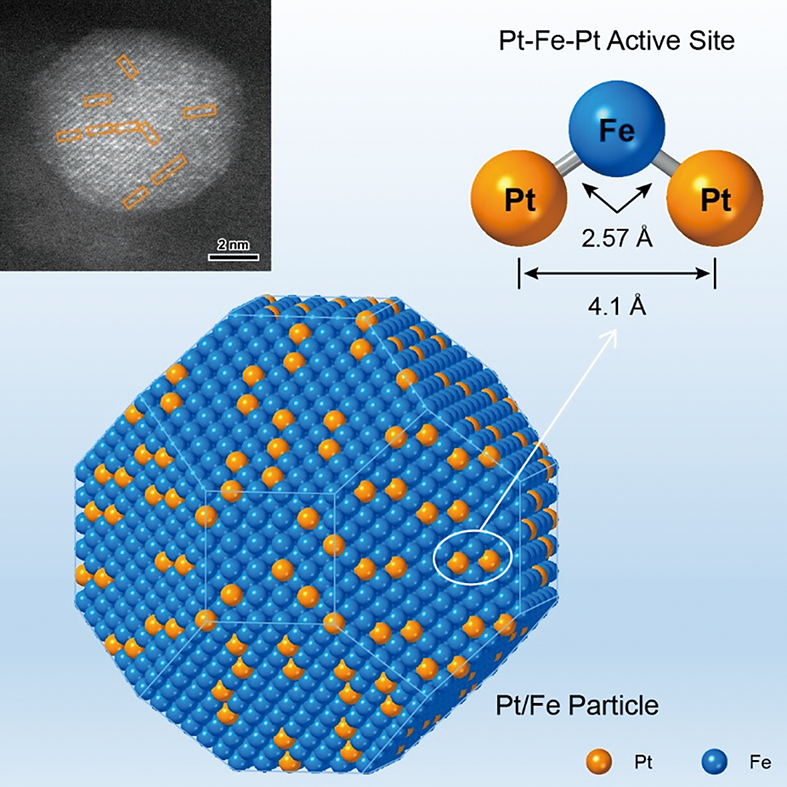
Tailoring active sites on bimetallic catalysts with atomic precision. (Graphic: ZHOU Di)

