When illuminated by visible light, this catalyst converts biomass-derived compounds to diesel-fuel precursors and hydrogen. (Credit: Dr. WANG Feng, DICP)
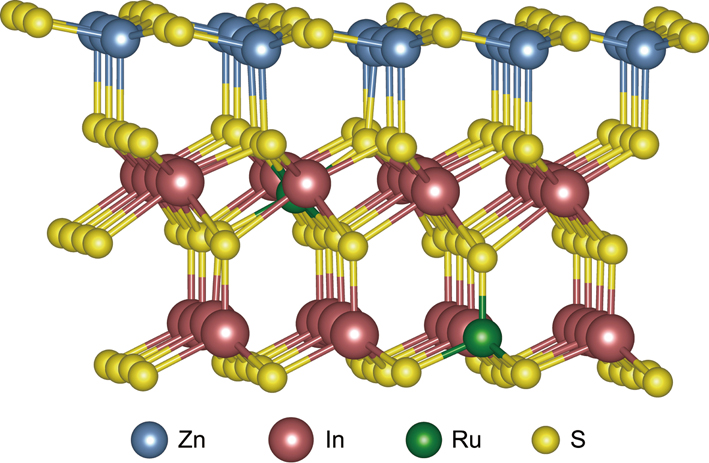
Harvesting light to split water into hydrogen (H2) and converting biomass to fuels or value-added chemicals are two hot pursuits in the field of green chemistry. Most efforts are focused on one topic or the other. In a recent study published in Nature Energy (doi: 10.1038/s41560-019-0403-5), Dr. WANG Feng’s group from the CAS Dalian Institute of Chemical Physics (DICP) reported a method for producing both diesel-fuel precursors and hydrogen from biomass-derived starting materials.
Using biomass in this fashion is attractive as it is the largest and the only sustainable carbon source whose use will not further mess up with our atmosphere by adding up the CO2 concentration like the fossil fuels did. More importantly, by coupling the production of two kinds of fuels with one catalyst, the Ru-doped ZnIn2S4, they solve a pending problem in photocatalytic H2 production. Conventionally, scientists use semiconductors as catalysts to produce the photoexcited electrons that then reduce protons in water to form hydrogen. This process also generates excited holes, or positive-charge carriers that may corrode the catalyst and reduce its efficacy gradually. One way out is by adding sacrificial reagents to quench these damaging charges. However, the use of sacrificial reagents could cause two new concerns: the generation of by-products that cause safety concern and wasting the oxidizing power of the excited holes for nothing value-added. So, it is of rational urge and practically value to make use of both excited electrons and holes and herein Dr. WANG’s group shows us a good example of how to make it happen by channeling these two kinds of powers for producing two kinds of fuels.
(By YAN Fusheng)

