Mapping the intricate journeys that cells take as they differentiate and mature is fundamental to understanding both normal development and disease progression. Now, scientists have devised an innovative computational method called PhyloVelo that generates detailed roadmaps to trace complex cell lineage trajectories. As reported in a recent Nature Biotechnology paper, PhyloVelo provides unprecedented insights into temporal cell state dynamics in diverse contexts from worm embryos to lung cancer.
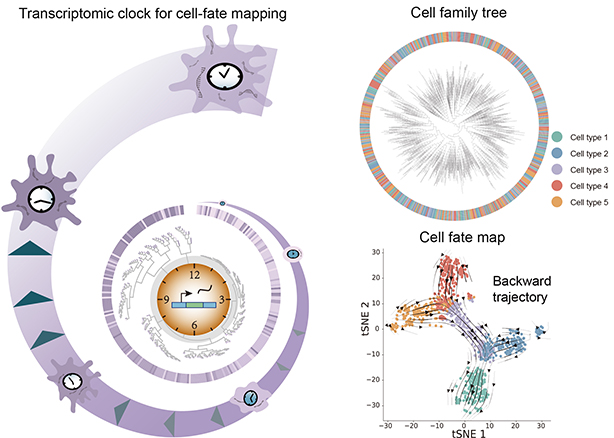
PhyloVelo, a new computational method that leverages monotonically expressed genes (MEGs) along cell divisions to quantify the transcriptomic velocity fields from lineage-resolved single-cell RNA-seq data, proves capable of mapping cellular journeys. (Credit: SIAT)
Organism development and disease progression both involve a series of cell-fate transitions that occur through repeated cell divisions. Essentially, all cells in an organism are related in a phylogenetic tree, where the root represents the fertilized egg or zygote. The branches of this tree signify cell divisions, and the leaves or endpoints depict mature cells that have adopted various final cell fates or types.
To understand how cell fate is determined during development and disease, it is crucial to elucidate two key factors: the precise order in which cell-states transition along the lineage, as well as the underlying gene regulatory mechanisms that drive these fate changes. Tracing both the sequence of events and the genomic factors that precipitate them will provide fundamental insights into cell differentiation.
Mapping cellular journeys is no easy feat. Methods like single-cell RNA sequencing can profile cell states but linking them into temporal sequences remains challenging. This is especially true in disease, where cell transitions can be abnormal. Another technique called RNA velocity uses RNA processing kinetics to predict future cell states from current data. However, RNA molecules are constantly being transcribed, spliced, and degraded inside cells. The timing of these processes varies and doesn’t follow constant rates assumed by the model. This mismatch between the model assumptions and real RNA behavior can lead to unreliable predictions.
Now the newly reported computational method PhyloVelo fills the gap. Developed by the joint team led by Dr. HU Zheng at the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences and Dr. ZHOU Da at the Xiamen University in China, this new pipeline can create roadmaps to trace complex cell lineage trajectories.
In this study, PhyloVelo integrates single-cell RNA-seq with genetic lineage tracing, which labels cell divisions with mutations. The number of mutations indicates time along the cell’s ancestral history.
One of the key innovations with PhyloVelo is identifying “monotonically expressed genes” (MEGs) that consistently increase or decrease over lineage time, which refers to sequence of cell divisions tracing back to the first progenitor cell or fertilized egg. More importantly and also interestingly, MEGs generalize across tissues and species, enriched in ribosome and translation genes. The expression levels of MEGs act as an internal clock, changing monotonically as cells move through lineage time. This allows PhyloVelo to align cells along a temporal trajectory to reconstruct their developmental path and pin down cell-state transition events.
Testing on simulations, worm and mouse embryos demonstrated PhyloVelo’s accuracy even with limited cells. It correctly recovered linear, branching and converging differentiation trajectories, outperforming the previously reported RNA velocity. The method revealed surprising dedifferentiation during lung cancer progression, with cells gaining stem-like behavior as tumors evolve. Analyses of other cancers and immune cells also provided unique insights.
When asked about future applications, Dr. HU commented that PhyloVelo could be applied to model dynamic processes like tumor evolution under drug treatment by utilizing lineage-traced single-cell RNA-seq data. He also envisioned using PhyloVelo to reveal principles governing cell-fate choices amidst genetic or environmental perturbations, such as genetic or epigenetic alterations, inflammatory responses, and tissue regeneration.
Dr. HU remarked that coupling lineage tracing with transcriptomics, though laborious, will be transformative. Further improved lineage tracing technologies and their application to human tissues would empower methods like PhyloVelo.
“Anticipating the ultimate cellular outcomes from their present states presents a formidable challenge. However, as an increasing wealth of lineage-annotated single-cell multiomic data is generated, the prospect of training a sophisticated computational model, such as an AI model, to predict cell fates gains traction,” envisioned Dr. HU when asked about the possibility to foresee cell destinations with PhyloVelo.
In summary, PhyloVelo integrates genetic lineage tracing with single-cell transcriptomics to create cell roadmaps illuminating both developmental and disease processes. By exploiting novel monotonically expressed gene clocks, it promises to reveal new biology and transform our understanding of cell fate determination.
Reference
Wang, K., Hou, L., Wang, X., Zhai, X., Lu, Z., Zi, Z., . . . Hu, Z. (2023). PhyloVelo enhances transcriptomic velocity field mapping using monotonically expressed genes. Nature Biotechnology. doi:10.1038/s41587-023-01887-5

