As revealed in a research published lately in Science, two human-origin monoclonal antibodies identified and reproduced by Chinese scientists were able to protect mice from COVID-19. These virus-neutralizing antibodies, acting to block the virus binding to its victim cells, were harvested from the blood of a convalescent donor once infected with COVID-19.
COVID-19, caused by the novel coronavirus SARS-CoV-2, has been rampaging globally and causing massive human death. The lucky ones who have managed to fight off the virus and survived the infection are walking gold mines in the eyes of scientists who are looking for neutralizing antibodies, a molecular weapon to hold back the virus attack by blocking COVID-19 virus bindings to host cells. These virus-targeting antibodies are the reason why the lucky ones are able to dodge the bullet.
The virus-targeting antibodies usually peak in the wake of infection and drop down when the battle is won. The direct screening of such antibodies from the blood is challenging, because it is very hard to pick them out, let alone getting them sequenced. A more feasible way is to acquire the genes encoding for such antibodies, because DNA sequencing is straightforward. By putting the antibody-encoding genes inside a host cell, such as yeast or insect cells, scientists can readily produce and harvest the desired antibody products from these cell factories. So, the production pipeline for virus-targeting antibodies is considered settled once their encoding genes are acquired.
The next thing is to find the virus-targeting antibody’s genes from the blood of a convalescent donor. Luckily, in the donor’s blood, there lingers some immune cells, called memory B-cells that carry in their DNA the genetic coding for the virus-targeting antibodies. These circulating memory B cells also carry “portraits” of virus-targeting antibodies at their surface, which can help them recognize the particular virus on their next encounter and spur themselves to produce a huge amount of protective virus-neutralizing antibodies. This is exactly the reason why the vaccine works by immunizing the body with particular antigens – usually by inactivated virus, viral proteins or agents that can produce such proteins – to incur certain memory B-cells. However, the research and development of effective and safe vaccines is a challenging task and takes time.
In the face of the current COVID-19 epidemic, particularly the grim prospects that the virus might stay with human beings for a long time, it is worth trying to develop virus-targeting antibodies. A recent effort of treasure-hunting for neutralizing antibodies from the blood of a donor, who has recovered from COVID-19, has proven to be rewarding.
A joint team of scientists, led by CAS Member George GAO from the CAS Institute of Microbiology, WU Yan from the Capital Medical University, GAO Feng from the CAS Tianjin Institute of Industrial Biotechnology and LIU Lei from the Shenzhen Third People’s Hospital, reported lately in Science that a noncompeting pair of neutralizing antibodies, harvested from the blood sample of a convalescent donor, can block the COVID-19 virus from binding to its receptor ACE2. Validated by a mouse model, these antibodies can effectively prohibit virus rampaging in the infected lungs.
ACE2, short for angiotensin-converting enzyme 2, is a cellular surface receptor that can be hijacked by the COVID-19 virus to make its entry into the cell. This receptor is abundant in the lining of blood vessels – this may also explain why the COVID-19 virus could cause widespread impacts on many different organs and tissues, which each needs a blood supply. The COVID-19 virus binds to ACE2 by using the spike glycoproteins on its surface. Specifically, it uses the receptor binding domain (RBD) of the spike, acting like tentacles, to grasp onto the cell receptor ACE2.
Based on the knowledge, the team expressed the COVID-19 virus RBD protein as bait to fish out specific single memory B-cells from the blood sample. These memory B-cells are the trophy earned by the donor from his/her once encounter with the COVID-19 virus and they carry the genetic codes for the neutralizing antibodies.
By pairing the genetic codes for the variable regions encoding the heavy and light chain – the two parts together deliver the binding ability of the antibodies, they successfully identified two human monoclonal antibodies (B38 and H4) that keep the virus from binding to its receptor ACE2. They also performed a competition assay and found that the two antibodies recognize different sites on RBD with partial overlap. This noncompeting feature of the two antibodies, as stated by the authors, could be a big plus in future clinical applications.
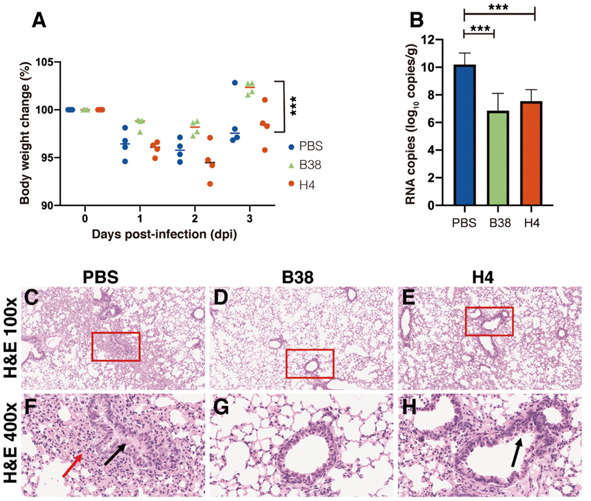
The protection efficiency of the two human monoclonal antibodies (B38 and H4) in hACE2 mice model post infection with the COVID-19 virus. (A) Body weight loss post infection for the PBS (control), B38 and H4 treatment groups. (B) Virus titers as indicated by viral RNA copies in the infected lungs of the three groups. (C to H) Representative histopathology of the lungs in COVID-19 virus infected hACE2 mice at 3 days post-infection. Compared to PBS (C and F, showing severe bronchopneumonia and interstitial pneumonia) and H4 groups (E and H, showing mild bronchopneumonia), viral challenged mice in the B38 group show no lesions (D and G). (Credit: Science)
Viruses tend to change themselves along each encounter with the host, particularly RNA viruses like SARS-CoV-2. This easy-changing feature of viruses is attributed to the fact that viral replication enzymes are relatively tolerant towards tiny errors that occur along replications of the viral genome. Some particular changes, e.g., the ones occurred to the virus RBD, could happen to destroy the binding of virus-neutralizing antibodies while maintaining its receptor binding for cell entry. As a result, the virus escapes the immune attack mobilized by the virus-neutralizing antibodies and get to ‘live’ another day. However, a double checkmate to the virus using a noncompeting pair of virus-targeting antibodies could greatly reduce the chance of virus immune escape, empowering a more potent protection.
They also conducted a therapeutic study in a mouse model to evaluate the protection efficacy of the two antibodies by giving the hACE2 transgenic mice a single dose of B38 or H4 12 hours after viral challenge. They found that the body weight of B38 group mice decreased slowly and recovered after 3 days exposure to the infection. They also measured the number of viral RNA copies in the lung on the third day of the exposure and found that the RNA copies of both B38 group and H4 group were significantly lower than the PBS group (the control), with a reduction of 32.8% and 26%, respectively. This in vivo study validates that these antibodies can curb virus propagation in infected lungs.
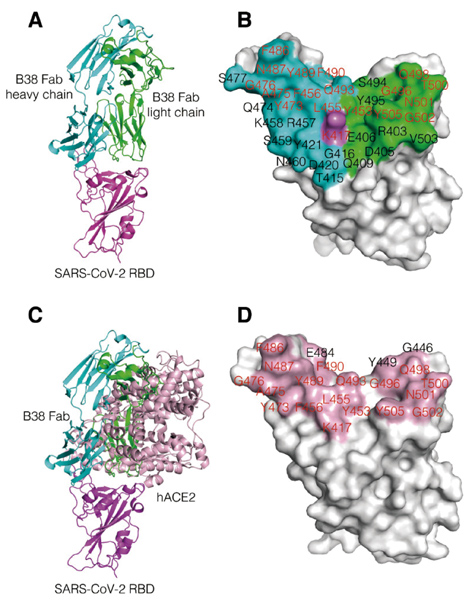
The overall structure of RBD-B38 complex (A) and the contact residues on RBD by B38 (B). Superimposition of B38/RBD and hACE2/RBD indicates their competitive binding to the virus (C). The contact residues on RBD by ACE2 are highlighted in light pink, in which the identical residues bind to both B38 and hACE2 are labeled in red (D). (Credit: Science)
To further explore the structural basis underlying these antibodies’ delivery of virus neutralization, the team revealed the crystal structures of the virus RBD and B38 complex. The RBD-B38 complex structure shows that most residues on the epitope overlap with the RBD-ACE2 binding interface, explaining the blocking effect and neutralizing capacity of B38.
“As the new epidemic continues to spread, the identification of viral RBD protein epitopes is crucial, which will provide valuable information for the development of vaccines. In addition, the molecular characteristics of neutralizing antibodies against epitopes help the development of small molecule or peptide drugs or inhibitors,” the study highlighted.
The scientists believe that the two neutralizing antibodies identified in this study are promising candidates for prophylactic and therapeutic treatment of COVID-19. The safety and efficacy of these virus-neutralizing antibodies for human use, however, remains to be confirmed before enlisting them to the therapeutic regimens.
Reference
Yan Wu*, Feiran Wang, Chenguang Shen, Weiyu Peng, Delin Li, Cheng Zhao, Zhaohui Li, Shihua Li, Yuhai Bi, Yang Yang, Yuhuan Gong, Haixia Xiao, Zheng Fan, Shuguang Tan, Guizhen Wu, Wenjie Tan, Xuancheng Lu, Changfa Fan, Qihui Wang, Yingxia Liu, Chen Zhang, Jianxun Qi, George Fu Gao*, Feng Gao*, Lei Liu*, (2020) A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science, eabc2241. doi: 10.1126/science.abc2241.

