By YAN Fusheng
For decades, lignin—the rigid polymer that gives plants their structure—has been the “problem child” of green chemistry. When processed, its molecules tangle like stubborn knots, forming useless clumps. Then came 2024’s transformative solution—breaking lignin’s tangles at the molecular level. In that May, a team led by scientists from the Chinese Academy of Sciences (CAS) came up with an approach to make this “knotty problem” profitable by hacking lignin’s chemistry to spin it into bio-bisphenols, sustainable building blocks for plastics. Online published in Nature, the study reimagines lignin not as waste but as a treasure trove for greener materials.
Steer the Chaos
Under acidic conditions, lignin generates reactive intermediates called benzylic carbocations—rogue molecules that latch onto anything nearby, creating a mess of crosslinks. Traditional “lignin-first” strategies tried to block these reactions, akin to putting a lid on a boiling pot. But researchers led by Dr. WANG Feng from the CAS Dalian Institute of Chemical Physics (DICP) and Dr. Joseph S. M. Samec from Stockholm University flipped the script: Why not steer the chaos instead?
By adding lignin-derived phenols like syringol (a compound found in oak barrels and smoked foods), the team created “molecular traps.” These phenols snatch the reactive carbocations mid-reaction, redirecting them to form orderly structures. This process, called arylation, attaches molecular “handles” to lignin, making it easier to dismantle later. As a result, lignin is transformed into syringolated lignin (S-lignin)—a modified polymer that’s primed for transformation.
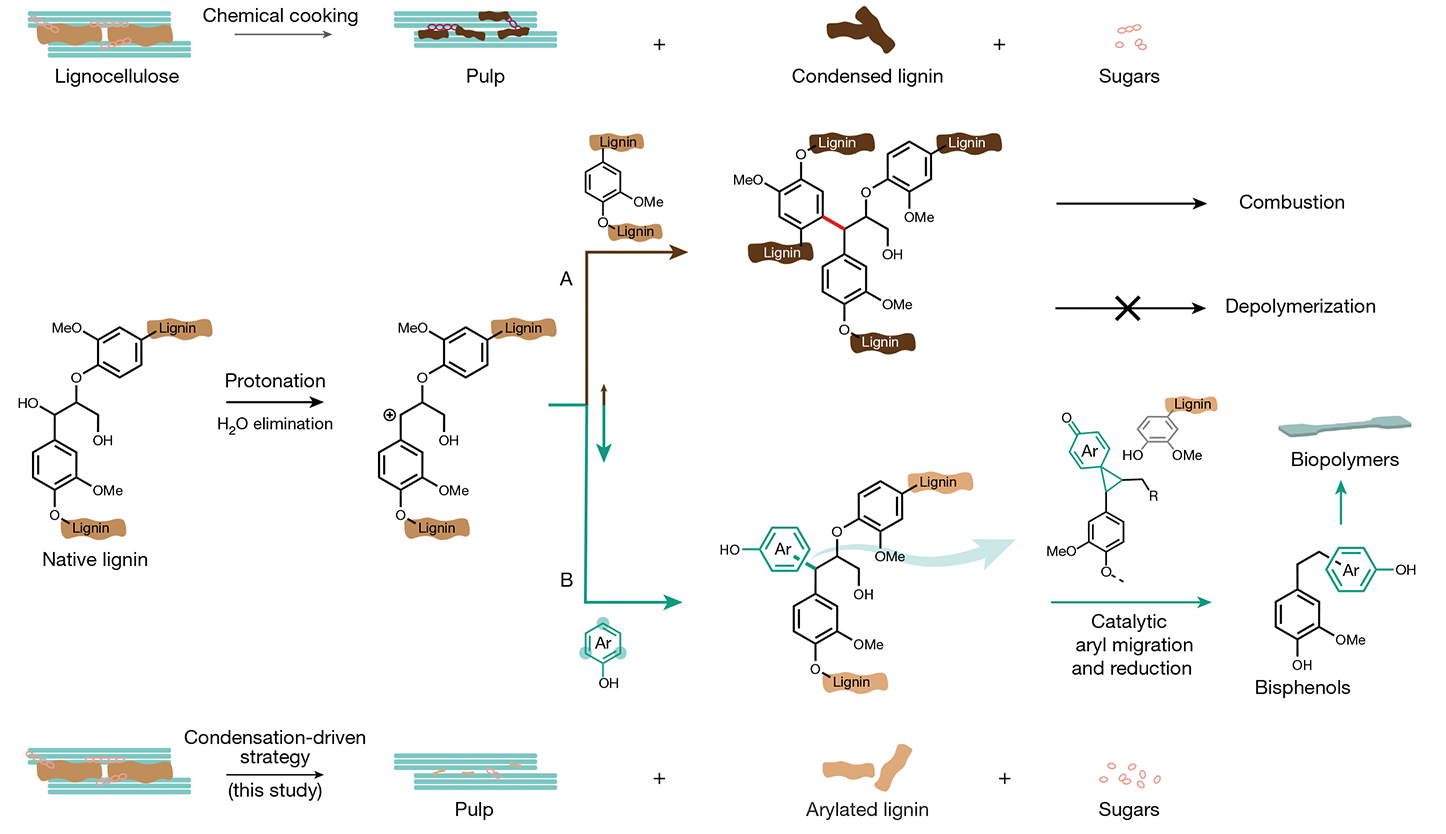
By adding phenolic additives (bottom, path B), this study redirects lignin’s fate from forming useless clumps and being burned
as waste to bisphenols—key green plastic precursors. (Graphic: Li et al., 2024)
The Catalytic Tango
Researchers developed an efficient method to transform S-lignin into valuable chemical products. The process involves two key chemical steps. First, they use p-toluenesulfonic acid (pTSA) as a catalyst to rearrange the structure of syringol molecules by relocating their aromatic rings. Following this, they employ palladium catalysts to perform controlled hydrogenation, which helps stabilize the newly formed molecular structures.
This tandem process converts up to 48% of lignin into bisphenols—compounds critical for making plastics. When tested on poplar wood, the method yielded delignified pulp (ideal for textiles) and xylose (a sugar used in biofuels).
Safer Plastics and Beyond
The star products—syringol-derived bisphenols (SLBPs) and phenol-derived bisphenols (PLBPs)—are designed to replace bisphenol A (BPA), a fossil-based plasticizer linked to hormone disruption. Unlike BPA’s rigid methylene bridge, SLBPs feature a flexible 1,2-diarylethane backbone, like swapping a steel beam for a shock absorber.
Lab tests revealed SLBPs reduced estrogenic activity by 110-fold compared to BPA, while PLBPs showed 27- to 52-fold reductions. So, it’s not just about replacing BPA. They’re building safer molecules from the ground up.
Apart from benign bisphenols, the method also allows for the production of multiple high-value products from lignocellulose. High-quality pulp, suitable for textiles and cellulose derivatives, is one such output. Xylose, another valuable component, can be converted into xylitol, a sugar substitute, or further processed into furans for biodegradable packaging. Additionally, lignin oligomers derived from the process can be utilized in resins and various other applications.
Lignin accounts for 30% of Earth’s renewable carbon, yet most is burned for low-value energy. This work cracks the code to upgrade it into high-performance, non-toxic materials.
While promising, scaling this process from lab benches to factories remains a high-stake puzzle. The study cautions that further investigation is necessary to optimize the catalytic process and ensure the sustainability of the reagents used.
Despite these hurdles, the study demonstrated a proof-of-concept approach that cracks open a new frontier for bio-based materials—one that could catalyze a long-awaited shift toward fossil-free polymer economies.
Reference
Li, N., Yan, K., Rukkijakan, T., et al. (2024) Selective lignin arylation for biomass fractionation and benign bisphenols. Nature, 630(8016), 381–386. doi:10.1038/s41586-024-07446-5

