By YAN Fusheng
The 2024 development of a precision-engineered retrotransposon system marked a significant milestone in mammalian genome-editing research. As appeared in the July 8 issue of Cell, this methodological breakthrough established a novel framework for site-specific gene delivery through repurposing ancient viral tools.
The Hidden World Within Our DNA
Our genomes are not static entities; they are dynamic, with a significant portion being composed of mobile genetic elements, including retrotransposons. These elements, often referred to as “jumping genes,” have the remarkable ability to copy themselves and insert these copies into new locations within the genome. While they have long been viewed as “selfish DNA” or genetic parasites, scientists are beginning to appreciate their critical roles in shaping genome evolution and function.
R2 retrotransposons, the focus of this study, are a particular type of non-LTR (long terminal repeat) retrotransposon that target specific sites in the genome, primarily ribosomal DNA (rDNA) loci. You can think of them as highly specialized genetic tools, designed by nature to operate with remarkable precision.
From “Jumping Genes” to Targeted Delivery Systems
The research team, led by Dr. LI Wei and CAS Member Dr. ZHOU Qi at the Institute of Zoology (IOZ) of the Chinese Academy of Sciences, took a novel approach by engineering R2 retrotransposons to act as a targeted gene delivery system.
They started by analyzing a variety of R2 elements from diverse species, identifying a few capable of inserting full-length genes into mammalian cells. This was not a simple cut-and-paste job. It required a deep understanding of the R2 machinery, particularly the proteins involved in the retrotransposition process—the “copy-paste” process of retrotransposons jumping from one place to another within the host genome using RNA as a middleman.
Here’s how the engineered R2 system works:
The researchers developed an innovative approach that leverages RNA as the primary genetic cargo, moving away from traditional DNA delivery methods. By using RNA, they significantly reduced the risk of unwanted immune responses and minimized the potential for random mutations. This RNA could serve as a precise blueprint for the therapeutic gene of interest.
At the heart of this system is the R2 retrotransposon, which has been carefully modified to serve as a delivery vehicle. This molecular courier is specifically programmed to integrate the genetic material into a particular genomic location known as the 28S rDNA site. What makes this approach unique is its highly targeted integration mechanism.
The engineered R2 system employs a sophisticated targeting strategy that works like a precise lock-and-key mechanism. The RNA contains a special sequence called the R2 Homology Arm (RHA), which is designed to recognize and bind to the 28S rDNA site with exceptional accuracy. This ensures that the therapeutic gene is inserted exactly where it is intended, minimizing potential off-target effects and improving the overall precision of the gene delivery process.
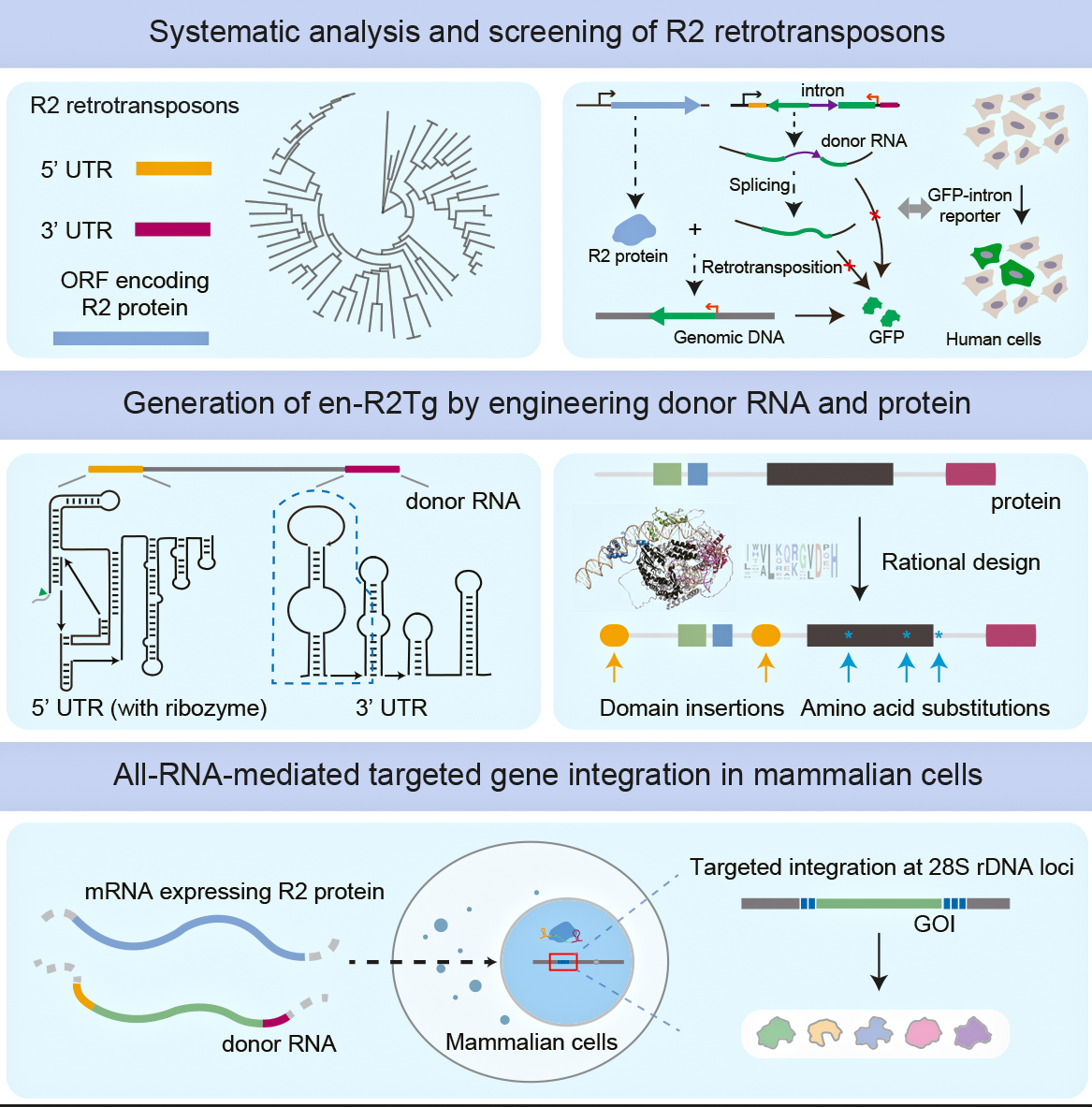
Engineered R2 retrotransposons enabled all-RNA-mediated, effective, and targeted gene integration in mammalian cells. (Graphic: IOZ)
Engineering R2 for Efficiency and Precision
The researchers didn’t stop at simply utilizing the natural R2 system. They further engineered both the R2 protein and the RNA donor to improve efficiency and precision. Using computational tools like AlphaFold, they predicted the 3D structure of the R2 protein and identified critical regions for its function. They introduced specific mutations in the protein and engineered the RNA to boost the integration activity.
The team discovered that the 5’ and 3’ UTRs (untranslated regions) of the R2 RNA play essential roles in the retrotransposition process. By modifying the structure of these regions, they were able to significantly increase the efficiency of gene integration. One critical component they engineered was an HDV-like ribozyme within the 5’ UTR, which acts like a self-cleaving scissor, enhancing the retrotransposition process. They also optimized the 3’ UTR for better activity.
They also incorporated other elements such as chromatin-modulating peptides (CMPs) to improve the R2’s access to the genome, and added elements to protect the synthesized cDNA and promote its integration. The final engineered R2 system, dubbed en-R2Tg, demonstrated remarkably high on-target integration specificity (99%). This is crucial, as off-target integration could lead to unwanted side effects.
The All-RNA System: A Safer and More Versatile Approach
One of the most exciting aspects of this research is the development of an all-RNA-mediated R2 system. This means that both the R2 protein and the donor RNA are delivered to the cells in the form of RNA. This RNA-based therapeutic approach offers several key benefits. RNA typically generates a lower immune response compared to DNA, which minimizes the risk of adverse immune reactions. By utilizing non-viral delivery methods like lipid nanoparticles (LNPs), which were used to deliver the mRNA vaccine against SARS-CoV-2, the approach provides a safer alternative to traditional viral vector-based gene therapies. Additionally, the all-RNA system demonstrates remarkable flexibility, enabling researchers to easily modify and target different therapeutic genes across various cell types.
The all-RNA en-R2Tg system showed impressive integration efficiency in multiple cell types. For instance, it achieved high integration efficiency in human primary T cells, which are notoriously difficult to genetically modify due to their sensitivity and the challenges of delivering genetic material without causing toxicity or cell death. The en-R2Tg system achieved efficient gene integration in these cells, which is a critical milestone for immunotherapy applications, such as CAR-T cell therapy. The ability to precisely insert therapeutic genes into T cells without disrupting their function opens the door to more effective and safer treatments for cancer and autoimmune diseases.
Besides, the all-RNA en-R2Tg system also performed well in embryonic gene editing. Achieving over 60% integration efficiency in mouse embryos, it can be a game-changer for developmental biology and gene therapy. This high efficiency means that the en-R2Tg system can be used to create transgenic animal models with high precision, reducing the time and cost associated with traditional methods like CRISPR-Cas9, which often require extensive screening to identify correctly edited embryos.
Safety and Long-Term Effects
While the potential of this technology is tremendous, it is essential to avoid potential harms produced by mistaken editing. Out of this concern, the researchers have taken great care to assess the specificity and precision of the en-R2Tg system.
Safety is a paramount consideration in this research. The engineered R2 system has demonstrated remarkable on-target integration specificity, meaning it can insert therapeutic genes at the desired location with high precision. Extensive testing revealed that off-target integration is rare, occurring in less than 1% of cases. Moreover, the en-R2Tg system produces far fewer unintended genetic errors compared to the wild-type R2 system, significantly reducing the risk of harmful insertions or deletions in the DNA.
Using advanced sequencing technologies, such as CRIT-seq to capture a detailed map of integration events, the team was able to track where and how well retrotransposons integrate into the genome. The results were promising, showing that the gene integration does not significantly disrupt normal gene expression. Encouraging long-term stability studies found that integrated genes remained stably expressed in cells for at least one month, suggesting potential for sustained therapeutic effects.
Despite these positive findings, the researchers maintain a responsible and transparent approach. They openly acknowledge that further research is necessary to comprehensively characterize the long-term effects of R2-mediated gene integration. Specific areas of continued investigation include mutation rate frequency and potential byproduct effects, ensuring a thorough and cautious approach to this innovative technology.
Future Directions and Broader Implications
This research opens a range of exciting possibilities in gene therapy. The ability to precisely deliver genes using an all-RNA system could be transformative for treating a variety of diseases, including genetic disorders, cancer, and infectious diseases. The authors also note that it could be combined with CRISPR-Cas technology to improve the programmability of the system, given that CRISPR-Cas is renowned for its ability to target specific DNA sequences with high precision.
This is a significant step forward in harnessing the power of “jumping genes” for therapeutic purposes. This study not only provides engineered R2 tools for targeted DNA integration but also offers crucial insights into further optimization of retrotransposon systems. In a sense, scientists are learning to reprogram these ancient genetic hitchhikers, transforming them from “selfish DNA” into powerful tools for healing.
The road ahead will undoubtedly involve rigorous testing and further refinement of this technology. But, if these initial results hold true, the future of medicine may well include the elegant repurposing of the dynamic genetic elements that dwell within us all.
Reference
Chen, Y., Luo, S., Hu, Y., et al. (2024) All-RNA-mediated targeted gene integration in mammalian cells with rationally engineered R2 retrotransposons. Cell, 187(17), 4674–4689.e4618. doi:10.1016/ j.cell.2024.06.020

