By YAN Fusheng (Staff Reporter)
Chromatin, a complex of DNA and proteins, makes up our chromosomes. When cells divide, the chromatin must be duplicated so that each new cell inherits a full set. A protein complex called chromatin assembly factor-1 (CAF-1) assembles chromatin onto newly copied DNA, but its mechanism is mysterious. Now, researchers from the CAS Institute of Biophysics (IBP) have solved critical parts of the CAF-1 puzzle using imaging techniques. Read on to learn how their findings unlock the secrets of this intricate molecular dance underlying faithful chromosome duplication during cell division.

The structural snapshots provide molecular insights into how chromatin assembly factor-1 (CAF-1) marshals histones and DNA into proper chromatin structure during the intricate chromatin assembly process that allows cells to accurately copy their genetic material. (Image by Science)
If you were to zoom into the inner world of a cell and examine its core, the nucleus, you would discover that DNA is not the sole inhabitant. Proteins called histones act like spools, wrapping up the long strands of DNA tightly into structures known as nucleosomes. Resembling beads, these nucleosomes represent the basic packaging units of DNA within chromatin. Connected to each other by 10-90 bp of linker DNA, nucleosomes give chromatin a “beads-on-a-string” structure when viewed under the microscope.
When cells divide, they must make copies of their DNA as well as rebuild the chromatin, so that each new cell gets a complete set of chromosomes. This process involves disassembling the nucleosomes ahead of the replication fork and then reassembling them behind it. The CAF-1 is critical for reassembling chromatin onto the newly copied DNA, but how it does the assembly job remained little known -- until very recently.
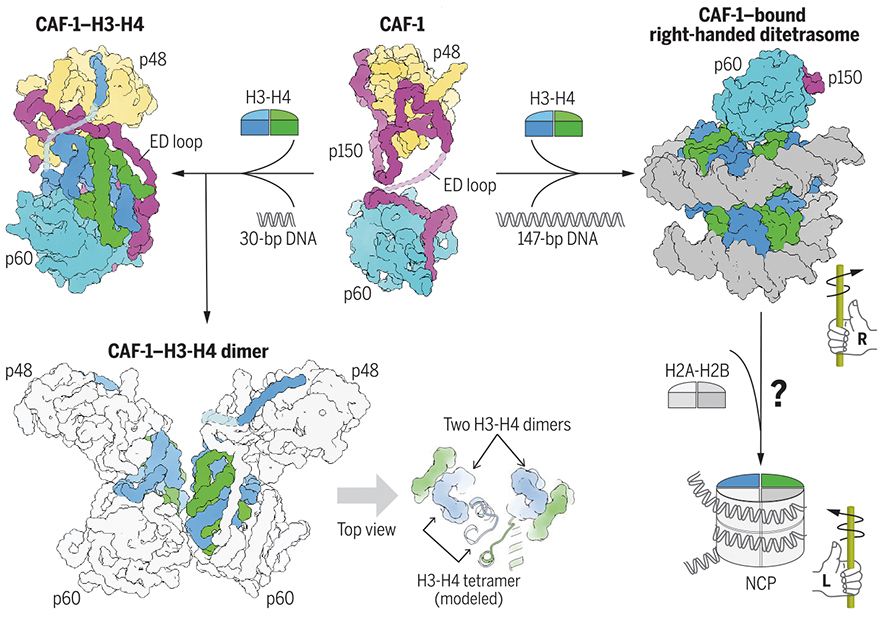
Researchers from the CAS Institute of Biophysics (IBP) solved key parts of the CAF-1 puzzle. Using x-ray crystallography and cryo-electron microscopy, they imaged CAF-1 both with and without two of its histone partners, H3 and H4. Their atomic-level snapshots reveal that CAF-1 is flexible, able to change its own shape as needed to grab histones and deposit them onto DNA during replication.
The snapshots also show how the different pieces of CAF-1 fit together and work with histones to assemble chromatin. They also show how CAF-1 opens up and secures histones H3-H4 for transport, preventing premature assembly, and then how DNA binding promotes CAF-1 dimerization to position histone dimers for tetramer formation, a key step in nucleosome creation.
The researchers found that CAF-1 has two globular “hands” connected by a floppy “arm.” The H3-H4 histone dimer binds by wedging between the separated hands, grabbing onto the central arm, which contains flexible amino acid loops ideal for wrapping around histones. CAF-1 binding covers up the part of H3-H4 that lets them join up into tetramers. When DNA is added, two CAF-1 complexes draw close together, correctly positioning the H3-H4 dimers so they can combine into tetramers. Additional biochemical experiments showed that DNA length affects CAF-1 dimerization and tetramer formation.
Surprisingly, CAF-1 appears to assemble an unusual right-handed intermediate containing H3-H4 tetramers before they wrap up DNA in the standard left-handed direction to form mature nucleosomes. The researchers speculate that this righty precursor could help alleviate torsional stress during replication, or assist with chromatin assembly at tricky chromosomal regions like centromeres.
By solving the long-mysterious structure of CAF-1 and capturing steps in the chromatin assembly process, this work opens up new avenues for understanding epigenetic inheritance and chromatin-mediated regulation of DNA replication and repair. These findings also offer clues to how chromatin assembly goes awry in various diseases. Moving forward, researchers can use the CAF-1 structures as guides for targeted experiments to further illuminate the intricate dance between DNA and histones in dividing cells.
Reference
Liu, C. P., Yu, Z., Xiong, J., Hu, J., Song, A., Ding, D., . . . Xu, R. M. (2023). Structural insights into histone binding and nucleosome assembly by chromatin assembly factor-1. Science, 381(6660), eadd8673. doi:10.1126/science.add8673

