In recent years, we have faced unprecedented threats by coronavirus (CoV) infections, particularly the ongoing COVID-19 pandemic. To fight back, scientists at the Institute of Microbiology, CAS (IMCAS) together with their collaborators developed a universal vaccine strategy against COVID-19, MERS, and SARS. Animal study showed promising results, and a vaccine candidate against COVID-19 based on this strategy has thereby proceeded to phase II clinical study.
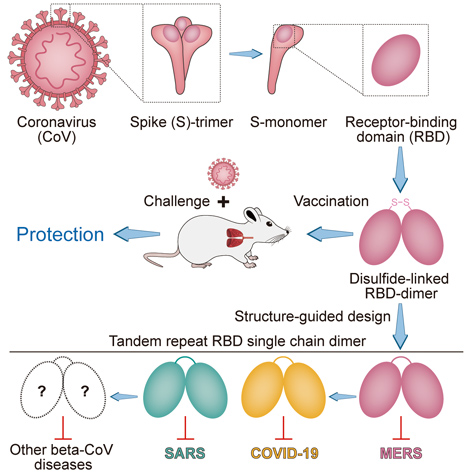
All coronaviruses (CoV) use a common weapon – the receptor-binding domain (RBD) on their spikes – for viral entry. Therefore, CoV RBD presents an attractive vaccine target. If designed alone as an immunogen for a vaccine, however, the CoV RBD monomer can only induce very limited antibody response, which might not be good enough to establish immunity against potential infection. This thankfully could be overcome by coupling two monomers side-by-side into an RBD dimer, which can protect immunized animals against life-threatening CoV diseases. (Image credit: Cell)
In retrospect, it seems that we have been waging an ever-going war against viruses only with a different enemy at various times. We have faced smallpox, poliovirus, Ebola, Spanish flu, dengue, rabies, HIV, Marburg virus, and the list keeps going on. We can now confidently confront smallpox, poliovirus and rabies; the wars with the most of them, however, are still in a deadlock, because an effective vaccine remains unavailable. Vaccine is still the major weapon we can depend and rely on to fight off life-threatening viruses.
In recent years, we have faced unprecedented threats by coronavirus (CoV) infections.
First identified in the 1960s, coronaviruses are named for the crown-like spikes on their surface. So far seven coronaviruses have been found being able to infect people. Four of them only cause mild cold-like illnesses. The remaining trios (SARS-CoV, MERS-CoV and SARS-CoV-2) are life-threatening and have caused a great deal of damage.
Late 2002, severe acute respiratory syndrome CoV (SARS-CoV) was first reported, and by the end of 2004 it had resulted in more than 8,000 infections worldwide with approximately 800 related deaths.
In 2012, Middle East respiratory syndrome CoV (MERS-CoV) emerged and was quickly spread into 27 countries, with a high fatality rate of 34.4%.
Currently, a coronavirus disease in 2019 (COVID-19) pandemic caused by SARS-CoV-2 infection is rampaging. Globally, as of 9:58am CEST, 10 July 2020, there have been 12,064,828 confirmed cases of COVID-19, including 550,384 deaths, reported to WHO.
The Looming Threats
“More and more SARS-related and MERS-related coronaviruses are identified in animal reservoir, raising the concerns for their zoonotic transmissions and pandemic potential in future,” said the authors from this reported study.
These lurking coronaviruses are like the Sword of Damocles, hanging over our heads. With the ability to evolve along infecting animals, the lurking ones may gain key mutations that enable them to recognize a particular cell receptor from a human cell. When it happens, the sword will drop again.
Anyway, COVID-19 pandemic is the matter of greatest urgency. The scientists worldwide are working on a solution. Still, no clinically effective drugs or vaccines are available. To put a stop to the ongoing COVID-19 pandemic, an effective vaccine is our best shot.
The Jewel in the Crown
We have now gained some important knowledge about these life-threatening coronaviruses. All of the three uses the receptor-binding domains (RBDs) at the far end of spikes for viral entry. Therefore, the CoV RBD presents an attractive vaccine target. When used as an immunogen to elicit immune protection, however, CoV RBD can only induce very limited antibody response due to its minor size, leaving a loophole for future possible invasion of viruses.
Using the whole S protein as an immunogen seems to be a good remedy, as its molecular size is large enough to elicit considerable immune response. Unfortunately, this would risk a backfire called antibody-dependent enhancement (ADE). When ADE occurs, the immunogen-induced poorly neutralizing antibodies (NAb) would become unwitting accomplices to help virus spreading, instead of stopping it. ADE has been previously observed for the feline CoV, and recently the SARS-CoV and MERS-CoV, raising a major concern in developing antibody-based therapy and vaccine development. The mechanism of how ADE occurs remains not fully understood.
Though ADE has up to now not been reported in COVID-19, it is worth the cautious effort to keep distance from this pitfall. One way to reduce the probability of ADE occurrence is picking an immunogen with dominant immune focusing. In this case, RBD is the Jewel in the crown. It seems we are back to square one: CoV RBD is an attractive vaccine target but with limited capability to elicit responsive antibodies.
Two Better than One
This time, a Chinese joint team led by GAO Fu, YAN Jinghua, QIN Chuan, and DAI Lianpan found a way out of this plight by making the CoV RBDs in dimers. They found that the RBD-dimers as immunogens are able to confer a more potent antibody response compared to the conventional RBD monomers.
Actually, the pursuit of this new strategy was started a couple of years ago when GAO’s team sought to develop a vaccine against MERS-CoV. They found that coupling two MERS-CoV RBD monomers via a disulfide bond could significantly enhance the antibody response and NAb induction. In a mouse infection model, this new design of “bilateral-lung-like” dimeric RBD antigen was shown to protect the immunized animals against MERS-CoV infection and greatly relieve lung injuries.
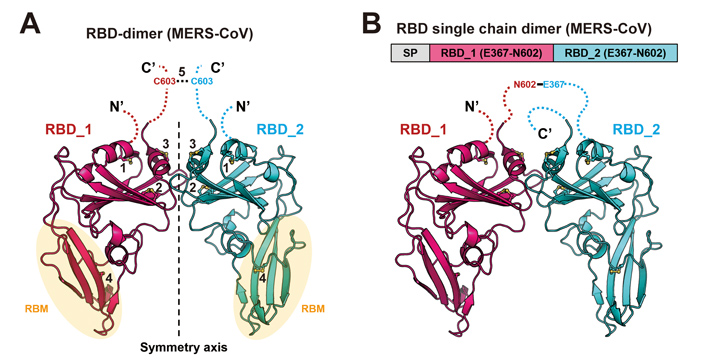
The two MERS-CoV RBD linked via a disulfide bond as “bilateral-lung” (left) or via tandem repeat (right). (Image credit: Cell
After obtaining these encouraging results, they further designed a more stable version of tandem repeat single chain dimer (sc-dimer). This time, two MERS-Cov RBD monomers are bridged by their own flexible terminal residues. Without introducing any exogenous sequence, RBD-sc-dimer is likely to lower the risk of undesired immune responses such as ADE. They found that the RBD-sc-dimer, as immunogen, can elicit strong antibody response as the disulfide-linked dimer.
Then, they extended this vaccine strategy to fight SARS-CoV-2 and SARS-CoV. They found that RBD-sc-dimers can significantly enhance the antigen-specific antibody responses compared to their conventional RBD-monomers, respectively. In a non-human primate infection model, the responsive protection was also observed in immunized rhesus monkeys (unpublished data).
In view of the ongoing COVID-19 pandemic and sporadic MERS outbreaks, the scientists also explored the feasibility to produce these protein subunit immunogens in an industry-standard cell system. While obtaining >98% purity, they successfully produced the RBD-sc-dimers at high-yields, to the level of gram per liter. Such high-yields highlight the scale-up production capacity to meet the global demands once their safety and effectiveness are validated in clinical trials.
On June 19, a vaccine candidate against COVID-19 was approved for phase I clinical study (Identifier: NCT04445194), entitled “A Multi-center, Double-blind, Randomized, Placebo Parallel Controlled, Safety and Tolerability Phase I Clinical Trial of Recombinant Novel Coronavirus Vaccine (CHO Cells) in Healthy People Between 18 and 59 Years of Age.” The Phase II clinical trial has been started on July 10, 2020, (Identifier: NCT04466085).
References
Lianpan Dai*, Tianyi Zheng, Kun Xu, Yuxuan Han, Lili Xu, Enqi Huang, Yaling An, Yingjie Cheng, Shihua Li, Mei Liu, Mi Yang, Yan Li, Huijun Cheng, Yuan Yuan, Wei Zhang, Changwen Ke, Gary
Wong, Jianxun Qi, Chuan Qin*, Jinghua Yan*, George F. Gao*, (2020) A universal design of betacoronavirus vaccines against COVID-19, MERS and SARS. Cell. doi: 10.1016/j.cell.2020.06.035.
Sheng-Fan Wang et al., (2014) Antibody-dependent sars coronavirus infection is mediated by antibodies against spike proteins. Biochemical and Biophysical Research Communications 451,
208. doi: 10.1016/j.bbrc.2014.07.090.
Yushun Wan et al., (2020) Molecular mechanism for antibody-dependent enhancement of coronavirus entry. Journal of Virology 94, e02015. doi: 10.1128/jvi.02015-19.

